Conserved Outer Tegument Component UL11 from Herpes Simplex Virus 1 Is an Intrinsically Disordered, RNA-Binding Protein
- PMID: 32371601
- PMCID: PMC7403781
- DOI: 10.1128/mBio.00810-20
Conserved Outer Tegument Component UL11 from Herpes Simplex Virus 1 Is an Intrinsically Disordered, RNA-Binding Protein
Abstract
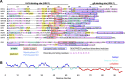
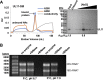
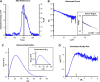
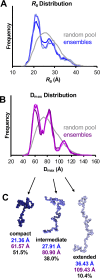
A distinguishing morphological feature of all herpesviruses is the multiprotein tegument layer located between the nucleocapsid and lipid envelope of the virion. Tegument proteins play multiple roles in viral replication, including viral assembly, but we do not yet understand their individual functions or how the tegument is assembled and organized. UL11, the smallest tegument protein, is important for several distinct processes in replication, including efficient virion morphogenesis and cell-cell spread. However, the mechanistic understanding of its role in these and other processes is limited in part by the scant knowledge of its biochemical and structural properties. Here, we report that UL11 from herpes simplex virus 1 (HSV-1) is an intrinsically disordered, conformationally dynamic protein that undergoes liquid-liquid phase separation (LLPS) in vitro Intrinsic disorder may underlie the ability of UL11 to exert multiple functions and bind multiple partners. Sequence analysis suggests that not only all UL11 homologs but also all HSV-1 tegument proteins contain intrinsically disordered regions of different lengths. The presence of intrinsic disorder, and potentially, the ability to form LLPS, may thus be a common feature of the tegument proteins. We hypothesize that tegument assembly may involve the formation of a biomolecular condensate, driven by the heterogeneous mixture of intrinsically disordered tegument proteins.IMPORTANCE Herpesvirus virions contain a unique tegument layer sandwiched between the capsid and lipid envelope and composed of multiple copies of about two dozen viral proteins. However, little is known about the structure of the tegument or how it is assembled. Here, we show that a conserved tegument protein UL11 from herpes simplex virus 1, a prototypical alphaherpesvirus, is an intrinsically disordered protein that undergoes liquid-liquid phase separation in vitro Through sequence analysis, we find intrinsically disordered regions of different lengths in all HSV-1 tegument proteins. We hypothesize that intrinsic disorder is a common characteristic of tegument proteins and propose a new model of tegument as a biomolecular condensate.
Keywords: RNA-binding protein; biomolecular condensate; conformational flexibility; herpesvirus; intrinsically disordered protein (IDP); liquid-liquid phase separation (LLPS); small‐angle X‐ray scattering (SAXS); structural model; tegument; viral assembly; viral protein.
Copyright © 2020 Metrick et al.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
