The Degree of Nesting between Spindles and Slow Oscillations Modulates Neural Synchrony
- PMID: 32371605
- PMCID: PMC7294805
- DOI: 10.1523/JNEUROSCI.2682-19.2020
The Degree of Nesting between Spindles and Slow Oscillations Modulates Neural Synchrony
Abstract
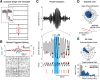
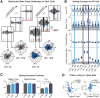
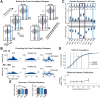
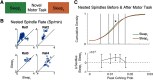
Spindles and slow oscillations (SOs) both appear to play an important role in memory consolidation. Spindle and SO "nesting," or the temporal overlap between the two events, is believed to modulate consolidation. However, the neurophysiological processes modified by nesting remain poorly understood. We thus recorded activity from the primary motor cortex of 4 male sleeping rats to investigate how SO and spindles interact to modulate the correlation structure of neural firing. During spindles, primary motor cortex neurons fired at a preferred phase, with neural pairs demonstrating greater neural synchrony, or correlated firing, during spindle peaks. We found a direct relationship between the temporal proximity between SO and spindles, and changes to the distribution of neural correlations; nesting was associated with narrowing of the distribution, with a reduction in low- and high-correlation pairs. Such narrowing may be consistent with greater exploration of neural states. Interestingly, after animals practiced a novel motor task, pairwise correlations increased during nested spindles, consistent with targeted strengthening of functional interactions. These findings may be key mechanisms through which spindle nesting supports memory consolidation.SIGNIFICANCE STATEMENT Our analysis revealed changes in cortical spiking structure that followed the waxing and waning of spindles; firing rates increased, spikes were more phase-locked to spindle-band local field potential, and synchrony across units peaked during spindles. Moreover, we showed that the degree of nesting between spindles and slow oscillations modified the correlation structure across units by narrowing the distribution of pairwise correlations. Finally, we demonstrated that engaging in a novel motor task increased pairwise correlations during nested spindles. These phenomena suggest key mechanisms through which the interaction of spindles and slow oscillations may support sensorimotor learning. More broadly, this work helps link large-scale measures of population activity to changes in spiking structure, a critical step in understanding neuroplasticity across multiple scales.
Keywords: correlation; sleep; slow oscillations; slow waves; spiking; spindle.
Copyright © 2020 the authors.
Figures


Similar articles
-
Coupling of Slow Oscillations in the Prefrontal and Motor Cortex Predicts Onset of Spindle Trains and Persistent Memory Reactivations.J Neurosci. 2024 Oct 23;44(43):e0621242024. doi: 10.1523/JNEUROSCI.0621-24.2024. J Neurosci. 2024. PMID: 39168655 Free PMC article.
-
Modulation of Neural Spiking in Motor Cortex-Cerebellar Networks during Sleep Spindles.eNeuro. 2024 May 6;11(5):ENEURO.0150-23.2024. doi: 10.1523/ENEURO.0150-23.2024. Print 2024 May. eNeuro. 2024. PMID: 38641414 Free PMC article.
-
Beyond spindles: interactions between sleep spindles and boundary frequencies during cued reactivation of motor memory representations.Sleep. 2018 Sep 1;41(9):zsy142. doi: 10.1093/sleep/zsy142. Sleep. 2018. PMID: 30137521 Free PMC article.
-
A sleep spindle framework for motor memory consolidation.Philos Trans R Soc Lond B Biol Sci. 2020 May 25;375(1799):20190232. doi: 10.1098/rstb.2019.0232. Epub 2020 Apr 6. Philos Trans R Soc Lond B Biol Sci. 2020. PMID: 32248783 Free PMC article. Review.
-
Hippocampal coupling with cortical and subcortical structures in the context of memory consolidation.Neurobiol Learn Mem. 2019 Apr;160:21-31. doi: 10.1016/j.nlm.2018.04.004. Epub 2018 Apr 13. Neurobiol Learn Mem. 2019. PMID: 29660400 Review.
Cited by
-
Slow oscillation-spindle coupling strength predicts real-life gross-motor learning in adolescents and adults.Elife. 2022 Feb 18;11:e66761. doi: 10.7554/eLife.66761. Elife. 2022. PMID: 35188457 Free PMC article.
-
Disturbed laterality of non-rapid eye movement sleep oscillations in post-stroke human sleep: a pilot study.Front Neurol. 2023 Nov 30;14:1243575. doi: 10.3389/fneur.2023.1243575. eCollection 2023. Front Neurol. 2023. PMID: 38099067 Free PMC article.
-
A design principle of spindle oscillations in mammalian sleep.iScience. 2022 Feb 5;25(3):103873. doi: 10.1016/j.isci.2022.103873. eCollection 2022 Mar 18. iScience. 2022. PMID: 35243235 Free PMC article.
-
Coupling between motor cortex and striatum increases during sleep over long-term skill learning.Elife. 2021 Sep 10;10:e64303. doi: 10.7554/eLife.64303. Elife. 2021. PMID: 34505576 Free PMC article.
-
Spindle oscillations in communicating axons within a reconstituted hippocampal formation are strongest in CA3 without thalamus.Sci Rep. 2024 Apr 10;14(1):8384. doi: 10.1038/s41598-024-58002-0. Sci Rep. 2024. PMID: 38600114 Free PMC article.
