MCL-1Matrix maintains neuronal survival by enhancing mitochondrial integrity and bioenergetic capacity under stress conditions
- PMID: 32371858
- PMCID: PMC7200794
- DOI: 10.1038/s41419-020-2498-9
MCL-1Matrix maintains neuronal survival by enhancing mitochondrial integrity and bioenergetic capacity under stress conditions
Abstract
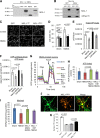
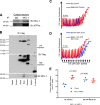
Mitochondria play a crucial role in neuronal survival through efficient energy metabolism. In pathological conditions, mitochondrial stress leads to neuronal death, which is regulated by the anti-apoptotic BCL-2 family of proteins. MCL-1 is an anti-apoptotic BCL-2 protein localized to mitochondria either in the outer membrane (OM) or inner membrane (Matrix), which have distinct roles in inhibiting apoptosis and promoting bioenergetics, respectively. While the anti-apoptotic role for Mcl1 is well characterized, the protective function of MCL-1 Matrix remains poorly understood. Here, we show MCL-1OM and MCL-1Matrix prevent neuronal death through distinct mechanisms. We report that MCL-1Matrix functions to preserve mitochondrial energy transduction and improves respiratory chain capacity by modulating mitochondrial oxygen consumption in response to mitochondrial stress. We show that MCL-1Matrix protects neurons from stress by enhancing respiratory function, and by inhibiting mitochondrial permeability transition pore opening. Taken together, our results provide novel insight into how MCL-1Matrix may confer neuroprotection under stress conditions involving loss of mitochondrial function.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

