Impact of corticosteroid therapy on outcomes of persons with SARS-CoV-2, SARS-CoV, or MERS-CoV infection: a systematic review and meta-analysis
- PMID: 32372026
- PMCID: PMC7199650
- DOI: 10.1038/s41375-020-0848-3
Impact of corticosteroid therapy on outcomes of persons with SARS-CoV-2, SARS-CoV, or MERS-CoV infection: a systematic review and meta-analysis
Abstract
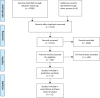
We performed a meta-analysis to determine safety and efficacy of corticosteroids in SARS-CoV-2, SARS-CoV, and MERS-CoV infections. We searched PubMed, Web of Science, Medline, WanFang Chinese database, and ZhiWang Chinese database using Boolean operators and search terms covering SARS-CoV-2, SARS-CoV, OR MERS-CoV AND corticosteroids to find appropriate studies. Review Manager 5.3 was used to analyze results of meta-analysis. Observational studies were analyzed for quality using the modified Newcastle-Ottawa scale and randomized clinical trials, using the Jadad scale. Subjects were divided into those with severe-only and other (severe and not severe) cohorts based on published criteria. Efficacy endpoints studied included mortality, hospitalization duration, rates of intensive care unit (ICU) admission, use of mechanical ventilation, and a composite endpoint (death, ICU admission, or mechanical ventilation). We included 11 reports including 10 cohort studies and 1 randomized clinical trial involving 5249 subjects (2003-2020). Two discussed the association of corticosteroids and virus clearing and 10 explored how corticosteroids impacted mortality, hospitalization duration, use of mechanical ventilation, and a composite endpoint. Corticosteroid use was associated with delayed virus clearing with a mean difference (MD) = 3.78 days (95% confidence Interval [CI] = 1.16, 6.41 days; I2 = 0%). There was no significant reduction in deaths with relative Risk Ratio (RR) = 1.07 (90% CI = 0.81; 1.42; I2 = 80%). Hospitalization duration was prolonged and use of mechanical ventilation increased. In conclusion, corticosteroid use in subjects with SARS-CoV-2, SARS-CoV, and MERS-CoV infections delayed virus clearing and did not convincingly improve survival, reduce hospitalization duration or ICU admission rate and/or use of mechanical ventilation. There were several adverse effects. Because of a preponderance of observational studies in the dataset and selection and publication biases our conclusions, especially regarding SARS-CoV-2, need confirmation in a randomized clinical trial. In the interim we suggest caution using corticosteroids in persons with COVID-19.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
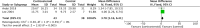

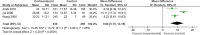
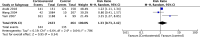
References
Publication types
MeSH terms
Substances
Grants and funding
- 81873428/National Natural Science Foundation of China (National Science Foundation of China)/International
- 81660682/National Natural Science Foundation of China (National Science Foundation of China)/International
- 2017ZT07S096/Guangdong Innovative and Entrepreneurial Research Team Program/International
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

