The coenzyme thiamine diphosphate displays a daily rhythm in the Arabidopsis nucleus
- PMID: 32372067
- PMCID: PMC7200797
- DOI: 10.1038/s42003-020-0927-z
The coenzyme thiamine diphosphate displays a daily rhythm in the Arabidopsis nucleus
Abstract
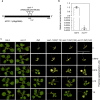
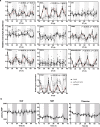
In plants, metabolic homeostasis-the driving force of growth and development-is achieved through the dynamic behavior of a network of enzymes, many of which depend on coenzymes for activity. The circadian clock is established to influence coordination of supply and demand of metabolites. Metabolic oscillations independent of the circadian clock, particularly at the subcellular level is unexplored. Here, we reveal a metabolic rhythm of the essential coenzyme thiamine diphosphate (TDP) in the Arabidopsis nucleus. We show there is temporal separation of the clock control of cellular biosynthesis and transport of TDP at the transcriptional level. Taking advantage of the sole reported riboswitch metabolite sensor in plants, we show that TDP oscillates in the nucleus. This oscillation is a function of a light-dark cycle and is independent of circadian clock control. The findings are important to understand plant fitness in terms of metabolite rhythms.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

