The evolution of strategies to minimise the risk of human drug-induced liver injury (DILI) in drug discovery and development
- PMID: 32372214
- PMCID: PMC7395068
- DOI: 10.1007/s00204-020-02763-w
The evolution of strategies to minimise the risk of human drug-induced liver injury (DILI) in drug discovery and development
Abstract
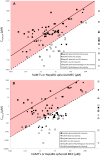
Early identification of toxicity associated with new chemical entities (NCEs) is critical in preventing late-stage drug development attrition. Liver injury remains a leading cause of drug failures in clinical trials and post-approval withdrawals reflecting the poor translation between traditional preclinical animal models and human clinical outcomes. For this reason, preclinical strategies have evolved over recent years to incorporate more sophisticated human in vitro cell-based models with multi-parametric endpoints. This review aims to highlight the evolution of the strategies adopted to improve human hepatotoxicity prediction in drug discovery and compares/contrasts these with recent activities in our lab. The key role of human exposure and hepatic drug uptake transporters (e.g. OATPs, OAT2) is also elaborated.
Keywords: Cmax,u; Cmax.tot; DILI; HCI; Hepatotoxicity; Spheroid; Strategies.
Conflict of interest statement
Paul Walker, Stephanie Ryder, Andrea Lavado, Clive Dilworth and Robert Riley, are employees past or present of Cyprotex Discovery Ltd, a company that develops and provides in vitro DILI assays mentioned in this review.
Figures


References
-
- Aleo MD, Luo Y, Swiss R, Bonin PD, Potter DM, Will Y. Human drug-induced liver injury severity is highly associated with dual inhibition of liver mitochondrial function and bile salt export pump. Hepatology. 2014;60(3):1015–1022. - PubMed
-
- Aleo MD, Shah F, Allen S, Barton HA, Costales C, Lazzaro S, Leung L, Nilson A, Obach RS, Rodrigues AD, Will Y. Moving beyond binary predictions of human drug-induced liver injury (DILI) toward contrasting relative risk potential. Chem Res Toxicol. 2019;33(1):223–238. - PubMed
-
- Albrecht W, Kappenberg F, Brecklinghaus T, Stoeber R, Marchan R, Zhang M, Ebbert K, Kirschner H, Grinberg M, Leist M, Moritz W, Cadenas C, Ghallab A, Reinders J, Vartak N, van Thriel C, Golka K, Tolosa L, Castell J, Damm G, Seehofer D, Lampen A, Braeuning A, Buhrke T, Behr AC, Oberemm A, Gu X, Kittana N, van de Water B, Kreiling R, Fayyaz S, van Aerts L, Smedsrød B, Ellinger-Ziegelbauer H, Steger-Hartmann T, Gundert-Remy U, Zeigerer A, Ullrich A, Runge D, Lee SML, Schiergens TS, Kuepfer L, Aguayo-Orozco A, Sachinidis A, Edlund K, Gardner I, Rahnenführer J, Hengstler JG. Prediction of human drug-induced liver injury (DILI) in relation to oral doses and blood concentrations. Arch Toxicol. 2019;93(6):1609–1637. - PubMed
-
- Aninat C, Piton A, Glaise D, Le Charpentier T, Langouët S, Morel F, Guguen-Guillouzo C, Guillouzo A. Expression of cytochromes P450, conjugating enzymes and nuclear receptors in human hepatoma HepaRG cells. Drug Metab Dispos. 2006;34:75–83. - PubMed
-
- Atienzar FA, Novik EI, Gerets HH, Parekh A, Delatour C, Cardenas A, MacDonald J, Yarmush ML, Dhalluin S. Predictivity of dog co-culture model, primary human hepatocytes and HepG2 cells for the detection of hepatotoxic drugs in humans. Tox and Appl Pharma. 2014;275:44–61. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

