Preventive effect of low-dose landiolol on postoperative atrial fibrillation study (PELTA study)
- PMID: 32372277
- PMCID: PMC7581600
- DOI: 10.1007/s11748-020-01364-9
Preventive effect of low-dose landiolol on postoperative atrial fibrillation study (PELTA study)
Abstract
Objective: To investigate the efficacy of prophylactic administration of low-dose landiolol on postoperative atrial fibrillation (POAF) in patients after cardiovascular surgery.
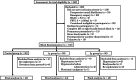
Methods: Consecutive 150 patients over 70 years of age who underwent cardiovascular surgery for valvular, ischemic heart, and aortic diseases were enrolled in this single-center prospective randomized control study from 2010 to 2014. They were assigned to three treatment groups: 1γ group (landiolol at 1 μg/kg/min), 2γ group (landiolol at 2 μg/kg/min), or control group (no landiolol). In the two landiolol groups, landiolol hydrochloride was intravenously administered for a period of 4 days postoperatively. Electrocardiography was continuously monitored during the study period, and cardiologists eventually assessed whether POAF occurred or not.
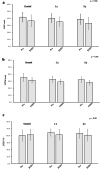
Results: POAF occurred in 24.4% of patients in the control group, 18.2% in 1γ group, and 11.1% in 2γ group (p = 0.256). Multivariate logistic regression analysis showed that the incidence of POAF tended to decrease depending on the dose of landiolol (trend-p = 0.120; 1γ group: OR = 0.786, 95% CI 0.257-2.404; 2γ group: OR = 0.379, 95% CI 0.112-1.287). Subgroup analysis showed a significant dose-dependent reduction in POAF among categories of female sex, non-use of angiotensin II receptor blockers (ARBs) before surgery, and valve surgery (each trend-p = 0.02, 0.03, and 0.004).
Conclusions: These findings indicate that prophylactic administration of low-dose landiolol may not be effective for preventing the occurrence of POAF in overall patients after cardiovascular surgery, but the administration could be beneficial to female patients, patients not using ARBs preoperatively, and those after valvular surgery.
Keywords: Cardiovascular surgery; Landiolol hydrochloride; Low dose; Postoperative atrial fibrillation; Prophylactic administration.
Conflict of interest statement
KK belonged to the Research Division of Sciences for Aortic Disease and his remuneration was derived from a donation to the division partially supported by a research grant from Ono Pharmaceutical Co., Ltd. YS is an affiliate of the Research Division of Sciences for Aortic Disease. The other authors declare that they have no conflict of interest.
Figures


References
-
- Jørgensen TH, Thygesen JB, Thyregod HG, Svendsen JH, Søndergaard L. New-onset atrial fibrillation after surgical aortic valve replacement and transcatheter aortic valve implantation: a concise review. J Invasive Cardiol. 2015;27:41–47. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

