Cerebellar Astrocyte Transduction as Gene Therapy for Megalencephalic Leukoencephalopathy
- PMID: 32372403
- PMCID: PMC7851290
- DOI: 10.1007/s13311-020-00865-y
Cerebellar Astrocyte Transduction as Gene Therapy for Megalencephalic Leukoencephalopathy
Abstract
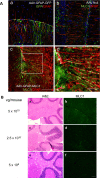
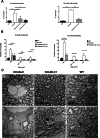
Megalencephalic leukoencephalopathy with subcortical cysts (MLC) is a rare genetic disorder belonging to the group of vacuolating leukodystrophies. It is characterized by megalencephaly, loss of motor functions, epilepsy, and mild mental decline. In brain biopsies of MLC patients, vacuoles were observed in myelin and in astrocytes surrounding blood vessels. There is no therapy for MLC patients, only supportive treatment. We show here a preclinical gene therapy approach for MLC using the Mlc1 knock-out mouse. An adeno-associated virus coding for human MLC1 under the control of the glial fibrillary acidic protein promoter was injected in the cerebellar subarachnoid space of Mlc1 knock-out and wild-type animals at 2 months of age, before the onset of the disease, as a preventive approach. We also tested a therapeutic strategy by injecting the animals at 5 months, once the histopathological abnormalities are starting, or at 15 months, when they have progressed to a more severe pathology. MLC1 expression in the cerebellum restored the adhesion molecule GlialCAM and the chloride channel ClC-2 localization in Bergmann glia, which both are mislocalized in Mlc1 knock-out model. More importantly, myelin vacuolation was extremely reduced in treated mice at all ages and correlated with the amount of expressed MLC1 in Bergmann glia, indicating not only the preventive potential of this strategy but also its therapeutic capacity. In summary, here we provide the first therapeutic approach for patients affected with MLC. This work may have also implications to treat other diseases affecting motor function such as ataxias.
Keywords: AAVrh10; Gene therapy; GlialCAM; MLC; cerebellum; myelin.
Figures





Similar articles
-
Gene therapy rescues brain edema and motor function in a mouse model of megalencephalic leukoencephalopathy with subcortical cysts.Mol Ther. 2025 Apr 2;33(4):1434-1448. doi: 10.1016/j.ymthe.2025.02.046. Epub 2025 Mar 5. Mol Ther. 2025. PMID: 40051162 Free PMC article.
-
Megalencephalic Leukoencephalopathy: Insights Into Pathophysiology and Perspectives for Therapy.Front Cell Neurosci. 2021 Jan 22;14:627887. doi: 10.3389/fncel.2020.627887. eCollection 2020. Front Cell Neurosci. 2021. PMID: 33551753 Free PMC article.
-
Megalencephalic leukoencephalopathy with subcortical cysts: A personal biochemical retrospective.Eur J Med Genet. 2018 Jan;61(1):50-60. doi: 10.1016/j.ejmg.2017.10.013. Epub 2017 Oct 25. Eur J Med Genet. 2018. PMID: 29079544 Review.
-
Mice with megalencephalic leukoencephalopathy with cysts: a developmental angle.Ann Neurol. 2015 Jan;77(1):114-31. doi: 10.1002/ana.24307. Epub 2014 Dec 4. Ann Neurol. 2015. PMID: 25382142
-
GPR37 Receptors and Megalencephalic Leukoencephalopathy with Subcortical Cysts.Int J Mol Sci. 2022 May 16;23(10):5528. doi: 10.3390/ijms23105528. Int J Mol Sci. 2022. PMID: 35628339 Free PMC article. Review.
Cited by
-
Draining the brain: Gene therapy reverses brain edema in mice with the leukodystrophy MLC.Mol Ther. 2025 May 7;33(5):1872-1873. doi: 10.1016/j.ymthe.2025.04.011. Epub 2025 Apr 19. Mol Ther. 2025. PMID: 40254020 No abstract available.
-
Megalencephalic leukoencephalopathy with subcortical cysts: a variant update and review of the literature.Front Genet. 2024 Feb 29;15:1352947. doi: 10.3389/fgene.2024.1352947. eCollection 2024. Front Genet. 2024. PMID: 38487253 Free PMC article.
-
Canavan Disease as a Model for Gene Therapy-Mediated Myelin Repair.Front Cell Neurosci. 2021 Apr 23;15:661928. doi: 10.3389/fncel.2021.661928. eCollection 2021. Front Cell Neurosci. 2021. PMID: 33967698 Free PMC article. Review.
-
Gene and Cellular Therapies for Leukodystrophies.Pharmaceutics. 2023 Oct 24;15(11):2522. doi: 10.3390/pharmaceutics15112522. Pharmaceutics. 2023. PMID: 38004502 Free PMC article. Review.
-
Gene therapy rescues brain edema and motor function in a mouse model of megalencephalic leukoencephalopathy with subcortical cysts.Mol Ther. 2025 Apr 2;33(4):1434-1448. doi: 10.1016/j.ymthe.2025.02.046. Epub 2025 Mar 5. Mol Ther. 2025. PMID: 40051162 Free PMC article.
References
-
- van der Knaap MS, Barth PG, Stroink H, et al. Leukoencephalopathy with swelling and a discrepantly mild clinical course in eight children. Ann Neurol. 1995;37:324–334. - PubMed
-
- Singhal BS, Gursahani RD, Udani VP, et al. Megalencephalic leukodystrophy in an Asian Indian ethnic group. Pediatr Neurol. 1996;14:291–296. - PubMed
-
- Ben-Zeev B, Gross V, Kushnir T, et al. Vacuolating megalencephalic leukoencephalopathy in 12 Israeli patients. J Child Neurol. 2001;16:93–99. - PubMed
-
- Harbord MG, Harden A, Harding B, et al. Megalencephaly with dysmyelination, spasticity, ataxia, seizures and distinctive neurophysiological findings in two siblings. Neuropediatrics. 1990;21:164–168. - PubMed

