Calcitonin gene-related peptide inhibits angiotensin II-induced NADPH oxidase-dependent ROS via the Src/STAT3 signalling pathway
- PMID: 32372557
- PMCID: PMC7294141
- DOI: 10.1111/jcmm.15288
Calcitonin gene-related peptide inhibits angiotensin II-induced NADPH oxidase-dependent ROS via the Src/STAT3 signalling pathway
Abstract
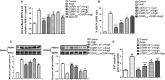
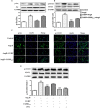
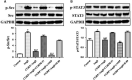
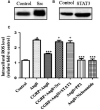
We had previously demonstrated that the calcitonin gene-related peptide (CGRP) suppresses the oxidative stress and vascular smooth muscle cell (VSMC) proliferation induced by vascular injury. A recent study also indicated that CGRP protects against the onset and development of angiotensin II (Ang II)-induced hypertension, vascular hypertrophy and oxidative stress. However, the mechanism behind the effects of CGRP on Ang II-induced oxidative stress is unclear. CGRP significantly suppressed the level of reactive oxygen species (ROS) generated by NADPH oxidase in Ang II-induced VSMCs. The Ang II-stimulated activation of both Src and the downstream transcription factor, STAT3, was abrogated by CGRP. However, the antioxidative effect of CGRP was lost following the expression of constitutively activated Src or STAT3. Pre-treatment with H-89 or CGRP8-37 also blocked the CGRP inhibitory effects against Ang II-induced oxidative stress. Additionally, both in vitro and in vivo analyses show that CGRP treatment inhibited Ang II-induced VSMC proliferation and hypertrophy, accompanied by a reduction in ROS generation. Collectively, these results demonstrate that CGRP exhibits its antioxidative effect by blocking the Src/STAT3 signalling pathway that is associated with Ang II-induced VSMC hypertrophy and hyperplasia.
Keywords: Ang II; CGRP; ROS; STAT3; Src; VSMCs.
© 2020 The Authors. Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures

Similar articles
-
Adrenomedullin inhibits angiotensin II-induced oxidative stress via Csk-mediated inhibition of Src activity.Am J Physiol Heart Circ Physiol. 2007 Apr;292(4):H1714-21. doi: 10.1152/ajpheart.00486.2006. Epub 2006 Oct 27. Am J Physiol Heart Circ Physiol. 2007. PMID: 17071733
-
Calcitonin gene-related peptide attenuates angiotensin II-induced ROS-dependent apoptosis in vascular smooth muscle cells by inhibiting the CaMKII/CREB signalling pathway.Biochem Biophys Res Commun. 2020 Jan 8;521(2):285-289. doi: 10.1016/j.bbrc.2019.10.064. Epub 2019 Oct 23. Biochem Biophys Res Commun. 2020. PMID: 31668374
-
Reactive oxygen species derived from NADPH oxidase 1 and mitochondria mediate angiotensin II-induced smooth muscle cell senescence.J Mol Cell Cardiol. 2016 Sep;98:18-27. doi: 10.1016/j.yjmcc.2016.07.001. Epub 2016 Jul 2. J Mol Cell Cardiol. 2016. PMID: 27381955
-
Angiotensin II, NADPH oxidase, and redox signaling in the vasculature.Antioxid Redox Signal. 2013 Oct 1;19(10):1110-20. doi: 10.1089/ars.2012.4641. Epub 2012 Jun 11. Antioxid Redox Signal. 2013. PMID: 22530599 Free PMC article. Review.
-
Angiotensin II and vascular injury.Curr Hypertens Rep. 2014 Jun;16(6):431. doi: 10.1007/s11906-014-0431-2. Curr Hypertens Rep. 2014. PMID: 24760441 Review.
Cited by
-
Calcitonin gene-related peptide protects from soluble fms-like tyrosine kinase-1-induced vascular dysfunction in a preeclampsia mouse model.Front Physiol. 2023 Aug 31;14:1221684. doi: 10.3389/fphys.2023.1221684. eCollection 2023. Front Physiol. 2023. PMID: 37719463 Free PMC article.
-
Oxidative Stress Activated by Sorafenib Alters the Temozolomide Sensitivity of Human Glioma Cells Through Autophagy and JAK2/STAT3-AIF Axis.Front Cell Dev Biol. 2021 Jun 14;9:660005. doi: 10.3389/fcell.2021.660005. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34277607 Free PMC article.
-
CGRP as a potential mediator for the sexually dimorphic responses to traumatic brain injury.Biol Sex Differ. 2024 May 30;15(1):44. doi: 10.1186/s13293-024-00619-x. Biol Sex Differ. 2024. PMID: 38816868 Free PMC article.
-
CGRP Inhibitors and Oxidative Stress Biomarkers in Resistant Migraine: A Real-Life Study with Erenumab, Fremanezumab, and Galcanezumab.J Clin Med. 2021 Oct 4;10(19):4586. doi: 10.3390/jcm10194586. J Clin Med. 2021. PMID: 34640604 Free PMC article.
-
The Crosstalk between Src and Hippo/YAP Signaling Pathways in Non-Small Cell Lung Cancer (NSCLC).Cancers (Basel). 2020 May 26;12(6):1361. doi: 10.3390/cancers12061361. Cancers (Basel). 2020. PMID: 32466572 Free PMC article. Review.
References
-
- Dzau VJ, Braun‐Dullaeus RC, Sedding DG. Vascular proliferation and atherosclerosis: new perspectives and therapeutic strategies. Nat Med. 2002;8:1249‐1256. - PubMed
-
- Kim S, Iwao H. Molecular and cellular mechanisms of angiotensin II‐mediated cardiovascular and renal diseases. Pharmacol Rev. 2000;52:11‐34. - PubMed
-
- Virdis A, Neves MF, Amiri F, Touyz RM, Schiffrin EL. Role of NAD(P)H oxidase on vascular alterations in angiotensin II‐infused mice. J Hypertens. 2004;22:535‐542. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

