Intravenous leiomyomatosis is inclined to a solid entity different from uterine leiomyoma based on RNA-seq analysis with RT-qPCR validation
- PMID: 32372565
- PMCID: PMC7333852
- DOI: 10.1002/cam4.3098
Intravenous leiomyomatosis is inclined to a solid entity different from uterine leiomyoma based on RNA-seq analysis with RT-qPCR validation
Abstract
Introduction: Intravenous leiomyomatosis (IVL) is currently regarded as a special variant of the common uterine leiomyoma (LM). Though IVL shows a similar histological morphology to LM, IVL is characterized by unique intravenous growth patterns and low-grade malignant potential, which are quite different from LM. There are currently few studies underlying the molecular alterations of IVL, though this information is important for understanding the pathogenesis of the disease, and for identifying potential biomarkers.
Method: We carried out a high-throughput whole transcriptome sequencing of tumor and normal tissue samples from five IVL patients and five LM patients and compared the differentially expressed genes (DEGs) between IVL and leiomyoma. We performed multiple different enrichment and target analyses, and the expression of selected DEGs was validated using RT-qPCR in formalin-fixed samples.
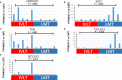
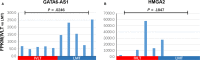
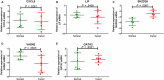
Results: Our study identified substantial different genes and pathways between IVL and LM, and functional enrichment analyses found several important pathways, such as angiogenesis and antiapoptosis pathways, as well as important related genes, including SH2D2A, VASH2, ADAM8, GATA2, TNF, and the lncRNA GATA6-AS1, as being significantly different between IVL and LM (P = .0024, P = .0195, P = .0212, P = .0435, P = .0401, and P = .0246, respectively). CXCL8, LIF, CDKN2A, BCL2A1, COL2A1, IGF1, and HMGA2 were also differently expressed between IVL and LM groups, but showed no statistical difference (P = .2409, P = .1773, P = .0596, P = .2737, P = .1553, P = .1045, and P = .1847, respectively) due to the large differences among individuals. Furthermore, RT-qPCR results for five selected DEGs in IVL tissues and adjacent nontumor tissues were mainly consistent with our sequencing results.
Conclusion: Our results indicated that IVL may be a solid entity that is unique and different from LM, proving consistent with previous studies. Furthermore, we identified DEGs, particularly within angiogenesis and antiapoptosis pathway-related genes that may play crucial roles in the development and pathogenesis of IVL and may be potential specific biomarkers.
Keywords: angiogenesis; differentially expressed genes; high-throughput whole transcriptome resequencing; intravenous leiomyomatosis.
© 2020 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Figures
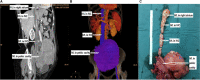
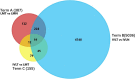
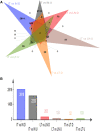
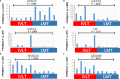
Similar articles
-
MED12 exon 2 mutation is uncommon in intravenous leiomyomatosis: clinicopathologic features and molecular study.Hum Pathol. 2020 May;99:36-42. doi: 10.1016/j.humpath.2020.03.011. Epub 2020 Mar 30. Hum Pathol. 2020. PMID: 32240666
-
Identification of the molecular relationship between intravenous leiomyomatosis and uterine myoma using RNA sequencing.Sci Rep. 2019 Feb 5;9(1):1442. doi: 10.1038/s41598-018-37452-3. Sci Rep. 2019. PMID: 30723247 Free PMC article.
-
Pathological and molecular insights into intravenous leiomyomatosis: an integrative multi-omics study.J Transl Med. 2025 Feb 26;23(1):229. doi: 10.1186/s12967-024-05919-9. J Transl Med. 2025. PMID: 40011937 Free PMC article.
-
Clinicopathologic features and clinical outcomes of intravenous leiomyomatosis of the uterus: A case series.Medicine (Baltimore). 2021 Jan 8;100(1):e24228. doi: 10.1097/MD.0000000000024228. Medicine (Baltimore). 2021. PMID: 33429819 Free PMC article. Review.
-
Pulmonary artery extension of uterine leiomyoma.J Card Surg. 2012 Jul;27(4):466-9. doi: 10.1111/j.1540-8191.2012.01469.x. Epub 2012 May 29. J Card Surg. 2012. PMID: 22640175 Review.
Cited by
-
Multimodality imaging applications in the diagnosis of and surgical treatment strategy for intravenous leiomyomatosis: a case description and literature analysis.Quant Imaging Med Surg. 2024 Jun 1;14(6):4281-4287. doi: 10.21037/qims-23-1772. Epub 2024 May 8. Quant Imaging Med Surg. 2024. PMID: 38846306 Free PMC article. No abstract available.
-
Intravenous leiomyomatosis: A case study and literature review.Radiol Case Rep. 2022 Sep 7;17(11):4203-4208. doi: 10.1016/j.radcr.2022.08.020. eCollection 2022 Nov. Radiol Case Rep. 2022. PMID: 36105826 Free PMC article.
-
Intravascular Leiomyoma Considered Preoperatively as Uterine Sarcoma: A Rare Case.Womens Health Rep (New Rochelle). 2024 Apr 4;5(1):334-339. doi: 10.1089/whr.2023.0091. eCollection 2024. Womens Health Rep (New Rochelle). 2024. PMID: 38596477 Free PMC article.
-
Intravascular leiomyomatosis in postmenopausal woman: a case report.Front Med (Lausanne). 2025 Mar 6;12:1517261. doi: 10.3389/fmed.2025.1517261. eCollection 2025. Front Med (Lausanne). 2025. PMID: 40115781 Free PMC article.
-
Update on clinical characteristics and molecular insights for uterine intravenous leiomyomatosis (Review).Oncol Lett. 2023 Nov 22;27(1):31. doi: 10.3892/ol.2023.14165. eCollection 2024 Jan. Oncol Lett. 2023. PMID: 38108079 Free PMC article. Review.
References
-
- Du J, Zhao X, Guo D, Li H, Sun B. Intravenous leiomyomatosis of the uterus: a clinicopathologic study of 18 cases, with emphasis on early diagnosis and appropriate treatment strategies. Hum Pathol. 2011;42:1240‐1246. - PubMed
-
- Wang WZ, Ma GT, Xiao Y, et al. Different expressions of Bcl‐2 and vascular endothelial growth factor Receptor‐3 in intravenous leiomyomatosis and classical leiomyoma. Med J Peking Union Med Coll Hosp. 2014;3:297‐301.
-
- Cramer SF, Patel A. The frequency of uterine leiomyomas. Am J Clin Pathol. 1990;94:435‐438. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

