The concordance of treatment decision guided by OncotypeDX and the PREDICT tool in real-world early-stage breast cancer
- PMID: 32372569
- PMCID: PMC7333833
- DOI: 10.1002/cam4.3088
The concordance of treatment decision guided by OncotypeDX and the PREDICT tool in real-world early-stage breast cancer
Abstract
Background: Decision-making regarding adjuvant chemotherapy for early-stage breast cancer can be guided by genomic assays such as OncotypeDX. The concordance of expected clinical decisions guided by OncotypeDX and prognostication online tools such as PREDICT is unknown.
Methods: We performed a retrospective single-center cohort study comprising all women with estrogen receptor (ER) positive, human epidermal growth factor receptor 2 (HER2) negative, node negative disease, whose tumors were sent for OncotypeDX analysis. Expected decision on adjuvant chemotherapy was evaluated using OncotypeDX and using PREDICT. The concordance between these two tools was calculated. The impact on concordance of prespecified features was assessed, including age, tumor size, intensity of ER and progesterone receptor (PR), grade, Ki67 and perineural and lymphovascular invasion.
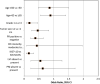
Results: A total of 445 women were included. Overall concordance was 75% (K = 0.284). The concordance was significantly higher for grade 1 disease compared to grade 2-3 (93% vs 72%, P < .001), tumor ≤ 1 cm compared to >1 cm (85% vs 72%, P = .009), PR positive compared to PR negative (78% vs 58%, P < .001) and ki67 < 10% compared to ≥10% (92% vs 63%, P < .001). The intensity of ER and the presence of perineural or lymphovascular invasion had no significant impact on concordance.
Conclusions: Compared to PREDICT, using OncotypeDx in node negative, ER positive disease is expected to change the clinical decision in a quarter of patients. The concordance between OncotypeDx and PREDICT is influenced by pathological features. In patients with very low risk, treatment decisions may be made based solely on clinical risk assessment.
Keywords: adjuvant; breast cancer; genomic assays; oncotype; predict tool.
© 2020 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
Moore declared
Figures
References
-
- DeSantis CE, Ma J, Goding Sauer A, et al. Breast cancer statistics, 2017, racial disparity in mortality by state. CA Cancer J Clin. 2017;67:439‐448. - PubMed
-
- Voduc KD, Cheang MC, Tyldesley S, et al. Breast cancer subtypes and the risk of local and regional relapse. J Clin Oncol. 2010;28:1684‐1691. - PubMed
-
- Olivotto IA, Bajdik CD, Ravdin PM, et al. Population‐based validation of the prognostic model ADJUVANT! for early breast cancer. J Clin Oncol. 2005;20:2716‐2725. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous