The role of the gut microbiome in sustainable teleost aquaculture
- PMID: 32372688
- PMCID: PMC7282919
- DOI: 10.1098/rspb.2020.0184
The role of the gut microbiome in sustainable teleost aquaculture
Abstract
As the most diverse vertebrate group and a major component of a growing global aquaculture industry, teleosts continue to attract significant scientific attention. The growth in global aquaculture, driven by declines in wild stocks, has provided additional empirical demand, and thus opportunities, to explore teleost diversity. Among key developments is the recent growth in microbiome exploration, facilitated by advances in high-throughput sequencing technologies. Here, we consider studies on teleost gut microbiomes in the context of sustainable aquaculture, which we have discussed in four themes: diet, immunity, artificial selection and closed-loop systems. We demonstrate the influence aquaculture has had on gut microbiome research, while also providing a road map for the main deterministic forces that influence the gut microbiome, with topical applications to aquaculture. Functional significance is considered within an aquaculture context with reference to impacts on nutrition and immunity. Finally, we identify key knowledge gaps, both methodological and conceptual, and propose promising applications of gut microbiome manipulation to aquaculture, and future priorities in microbiome research. These include insect-based feeds, vaccination, mechanism of pro- and prebiotics, artificial selection on the hologenome, in-water bacteriophages in recirculating aquaculture systems (RAS), physiochemical properties of water and dysbiosis as a biomarker.
Keywords: aquaculture; fish; gut; microbiome; review; teleost.
Conflict of interest statement
No competing interests.
Figures



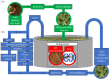
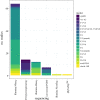
Similar articles
-
Influence of Fishmeal-Free Diets on Microbial Communities in Atlantic Salmon (Salmo salar) Recirculation Aquaculture Systems.Appl Environ Microbiol. 2016 Jul 15;82(15):4470-4481. doi: 10.1128/AEM.00902-16. Print 2016 Aug 1. Appl Environ Microbiol. 2016. PMID: 27129964 Free PMC article.
-
Interpopulation Variation in the Atlantic Salmon Microbiome Reflects Environmental and Genetic Diversity.Appl Environ Microbiol. 2018 Aug 1;84(16):e00691-18. doi: 10.1128/AEM.00691-18. Print 2018 Aug 15. Appl Environ Microbiol. 2018. PMID: 29915104 Free PMC article.
-
Harnessing the fish gut microbiome and immune system to enhance disease resistance in aquaculture.Fish Shellfish Immunol. 2025 Aug;163:110394. doi: 10.1016/j.fsi.2025.110394. Epub 2025 May 9. Fish Shellfish Immunol. 2025. PMID: 40350102 Review.
-
Aquaculture industry prospective from gut microbiome of fish and shellfish: An overview.J Anim Physiol Anim Nutr (Berl). 2022 Mar;106(2):441-469. doi: 10.1111/jpn.13619. Epub 2021 Aug 6. J Anim Physiol Anim Nutr (Berl). 2022. PMID: 34355428 Review.
-
Teleost microbiomes: the state of the art in their characterization, manipulation and importance in aquaculture and fisheries.Front Microbiol. 2014 Jun 2;5:207. doi: 10.3389/fmicb.2014.00207. eCollection 2014. Front Microbiol. 2014. PMID: 24917852 Free PMC article. Review.
Cited by
-
Functional feeds marginally alter immune expression and microbiota of Atlantic salmon (Salmo salar) gut, gill, and skin mucosa though evidence of tissue-specific signatures and host-microbe coadaptation remain.Anim Microbiome. 2022 Mar 10;4(1):20. doi: 10.1186/s42523-022-00173-0. Anim Microbiome. 2022. PMID: 35272695 Free PMC article.
-
The Impact of Tank Disinfectants on the Development of Microbiota in Gilthead Seabream (Sparus aurata) Larviculture Systems.Microorganisms. 2025 Jun 11;13(6):1359. doi: 10.3390/microorganisms13061359. Microorganisms. 2025. PMID: 40572247 Free PMC article.
-
Genome-resolved metagenomics suggests a mutualistic relationship between Mycoplasma and salmonid hosts.Commun Biol. 2021 May 14;4(1):579. doi: 10.1038/s42003-021-02105-1. Commun Biol. 2021. PMID: 33990699 Free PMC article.
-
Effects of Thermal Stress on the Gut Microbiome of Juvenile Milkfish (Chanos chanos).Microorganisms. 2020 Dec 22;9(1):5. doi: 10.3390/microorganisms9010005. Microorganisms. 2020. PMID: 33375015 Free PMC article.
-
Effect of intestinal microbiota on growth rate of Babylonia areolata.PLoS One. 2025 May 7;20(5):e0322985. doi: 10.1371/journal.pone.0322985. eCollection 2025. PLoS One. 2025. PMID: 40334216 Free PMC article.
References
-
- Whipp J, Lewis K, Cooke R. 1987. Fungi in biological control systems. Manchester, UK: Manchester University Press.
-
- Reuters T. 2012. Web of Science Service for UK Education. See https://wok.mimas.ac.uk.
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Research Materials

