Altered Sexual Behavior in Dopamine Transporter (DAT) Knockout Male Rats: A Behavioral, Neurochemical and Intracerebral Microdialysis Study
- PMID: 32372926
- PMCID: PMC7185326
- DOI: 10.3389/fnbeh.2020.00058
Altered Sexual Behavior in Dopamine Transporter (DAT) Knockout Male Rats: A Behavioral, Neurochemical and Intracerebral Microdialysis Study
Abstract
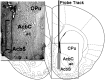
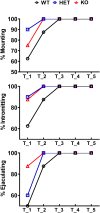
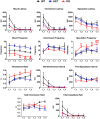
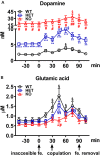
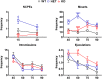
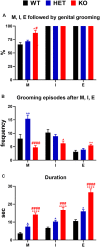
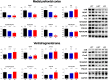
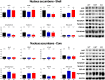
Central dopamine plays a key role in sexual behavior. Recently, a Dopamine Transporter knockout (DAT KO) rat has been developed, which displays several behavioral dysfunctions that have been related to increased extracellular dopamine levels and altered dopamine turnover secondary to DAT gene silencing. This prompted us to characterize the sexual behavior of these DAT KO rats and their heterozygote (HET) and wild type (WT) counterparts in classical copulatory tests with a sexually receptive female rat and to verify if and how the acquisition of sexual experience changes along five copulatory tests in these rat lines. Extracellular dopamine and glutamic acid concentrations were also measured in the dialysate obtained by intracerebral microdialysis from the nucleus accumbens (Acb) shell of DAT KO, HET and WT rats, which underwent five copulatory tests, when put in the presence of an inaccessible sexually receptive female rat and when copulation was allowed. Markers of neurotropism (BDNF, trkB), neural activation (Δ-FosB), functional (Arc and PSA-NCAM) and structural synaptic plasticity (synaptophysin, syntaxin-3, PSD-95) were also measured in the ventral tegmental area (VTA), Acb (shell and core) and medial prefrontal cortex (mPFC) by Western Blot assays. The results indicate that the sexual behavior of DAT KO vs. HET and WT rats shows peculiar differences, mainly due to a more rapid acquisition of stable sexual activity levels and to higher levels of sexual motivation and activity. These differences occurred with differential changes in dopamine and glutamic acid concentrations in Acb dialysates during sexual behavior, with lower increases of dopamine and glutamic acid in DAT KO vs. WT and HET rats, and a lower expression of the markers investigated, mainly in the mPFC, in DAT KO vs. WT rats. Together these findings confirm a key role of dopamine in sexual behavior and provide evidence that the permanently high levels of dopamine triggered by DAT gene silencing cause alterations in both the frontocortical glutamatergic neurons projecting to the Acb and VTA and in the mesolimbic dopaminergic neurons, leading to specific brain regional changes in trophic support and neuroplastic processes, which may have a role in the sexual behavior differences found among the three rat genotypes.
Keywords: Arc; BDNF/trkB; D-FosB; DAT knockout rats; dopamine; glutamic acid; sexual behavior; synaptic proteins.
Copyright © 2020 Sanna, Bratzu, Serra, Leo, Quartu, Boi, Espinoza, Gainetdinov, Melis and Argiolas.
Figures
Similar articles
-
c-Fos, ΔFosB, BDNF, trkB and Arc Expression in the Limbic System of Male Roman High- and Low-Avoidance Rats that Show Differences in Sexual Behavior: Effect of Sexual Activity.Neuroscience. 2019 Jan 1;396:1-23. doi: 10.1016/j.neuroscience.2018.11.002. Epub 2018 Nov 10. Neuroscience. 2019. PMID: 30423358
-
Dopamine, Noradrenaline and Differences in Sexual Behavior between Roman High and Low Avoidance Male Rats: A Microdialysis Study in the Medial Prefrontal Cortex.Front Behav Neurosci. 2017 Jun 7;11:108. doi: 10.3389/fnbeh.2017.00108. eCollection 2017. Front Behav Neurosci. 2017. PMID: 28638325 Free PMC article.
-
Involvement of dopamine in the differences in sexual behaviour between Roman high and low avoidance rats: an intracerebral microdialysis study.Behav Brain Res. 2015 Mar 15;281:177-86. doi: 10.1016/j.bbr.2014.12.009. Epub 2014 Dec 10. Behav Brain Res. 2015. PMID: 25497705
-
The endogenous cannabinoid system modulates male sexual behavior expression.Front Behav Neurosci. 2023 May 31;17:1198077. doi: 10.3389/fnbeh.2023.1198077. eCollection 2023. Front Behav Neurosci. 2023. PMID: 37324524 Free PMC article. Review.
-
Dopamine, Erectile Function and Male Sexual Behavior from the Past to the Present: A Review.Brain Sci. 2022 Jun 24;12(7):826. doi: 10.3390/brainsci12070826. Brain Sci. 2022. PMID: 35884633 Free PMC article. Review.
Cited by
-
Evaluation of Approach to a Conspecific and Blood Biochemical Parameters in TAAR1 Knockout Mice.Brain Sci. 2022 May 8;12(5):614. doi: 10.3390/brainsci12050614. Brain Sci. 2022. PMID: 35625001 Free PMC article.
-
Patterns of ethanol intake in male rats with partial dopamine transporter deficiency.Genes Brain Behav. 2023 Dec;22(6):e12847. doi: 10.1111/gbb.12847. Epub 2023 Jul 17. Genes Brain Behav. 2023. PMID: 37461188 Free PMC article.
-
Keeping Track of the Genealogy of Heterozygotes Using Epigenetic Reference Codes and Breeding Tables.Front Behav Neurosci. 2022 Feb 11;15:781235. doi: 10.3389/fnbeh.2021.781235. eCollection 2021. Front Behav Neurosci. 2022. PMID: 35221940 Free PMC article.
-
Glans penis electric stimulation modulates cerebral activity and functional connectivity in lifelong premature ejaculation revealed by functional MRI.Sci Rep. 2025 Jul 2;15(1):23328. doi: 10.1038/s41598-025-03994-6. Sci Rep. 2025. PMID: 40603932 Free PMC article.
-
Neurophysiology of male sexual arousal-Behavioral perspective.Front Behav Neurosci. 2024 Jan 24;17:1330460. doi: 10.3389/fnbeh.2023.1330460. eCollection 2023. Front Behav Neurosci. 2024. PMID: 38333545 Free PMC article. Review.
References
-
- Adinolfi A., Zelli S., Leo D., Carbone C., Mus L., Illiano P., et al. . (2019). Behavioral characterization of DAT-KO rats and evidence of asocial-like phenotypes in DAT-HET rats: the potential involvement of norepinephrine system. Behav. Brain Res. 359, 516–527. 10.1016/j.bbr.2018.11.028 - DOI - PubMed
-
- Apryatin S. A., Shipelin V. A., Trusov N. V., Mzhelskaya K. V., Evstratova V. S., Kirbaeva N. V., et al. . (2019). Comparative analysis of the influence of a high-fat/high-carbohydrate diet on the level of anxiety and neuromotor and cognitive functions in Wistar and DAT-KO rats. Physiol. Rep. 7:e13987. 10.14814/phy2.13987 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

