Danqi Pill Protects Against Heart Failure Post-Acute Myocardial Infarction via HIF-1α/PGC-1α Mediated Glucose Metabolism Pathway
- PMID: 32372956
- PMCID: PMC7187888
- DOI: 10.3389/fphar.2020.00458
Danqi Pill Protects Against Heart Failure Post-Acute Myocardial Infarction via HIF-1α/PGC-1α Mediated Glucose Metabolism Pathway
Abstract
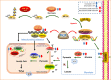
Aim: Heart failure (HF) post-acute myocardial infarction (AMI) leads to a large number of hospitalizations and deaths worldwide. Danqi pill (DQP) is included in the 2015 national pharmacopoeia and widely applied in the treatment of HF in clinics in China. We examined whether DQP acted on glucose metabolism to protect against HF post-AMI via hypoxia inducible factor-1 alpha (HIF-1α)/peroxisome proliferator-activated receptor α co-activator (PGC-1α) pathway.
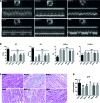
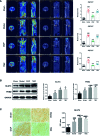
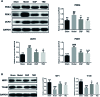
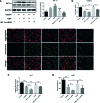
Methods and results: In this study, left anterior descending (LAD) artery ligation induced HF post-AMI rats and oxygen-glucose deprivation-reperfusion (OGD/R)-induced H9C2 cell model were structured to explore the efficacy and mechanism of DQP. Here we showed that DQP protected the heart against ischemic damage as evidenced by improved cardiac functions and attenuated inflammatory infiltration. The expressions of critical proteins involved in glucose intake and transportation such as GLUT4 and PKM2 were up-regulated, while negative regulatory proteins involved in oxidative phosphorylation were attenuated in the treatment of DQP. Moreover, DQP up-regulated NRF1 and TFAM, promoted mitochondrial biogenesis and increased myocardial adenosine triphosphate (ATP) level. The protection effects of DQP were significantly compromised by HIF-1α siRNA, suggesting that HIF-1α signaling pathway was the potential target of DQP on HF post-AMI.
Conclusions: DQP exhibits the efficacy to improve myocardial glucose metabolism, mitochondrial oxidative phosphorylation and biogenesis by regulating HIF-1α/PGC-1α signaling pathway in HF post-AMI rats.
Keywords: Danqi Pill (DQP); HIF-1α/PGC-1α pathway; acute myocardial infarction (AMI); glucose metabolism; heart failure (HF).
Copyright © 2020 Zhang, Guo, Wang, Wang, Wang, Wu, Li, Wang and Wang.
Figures

References
-
- Ambrose L. J., Abd-Jamil A. H., Gomes R. S., Carter E. E., Carr C. A., Clarke K., et al. (2014). Investigating mitochondrial metabolism in contracting HL-1 cardiomyocytes following hypoxia and pharmacological HIF activation identifies HIF-dependent and independent mechanisms of regulation. J. Cardiovasc. Pharmacol. Ther. 19, 574–585. 10.1177/1074248414524480 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

