Traditional Chinese Medicine Combined With Chemotherapy and Cetuximab or Bevacizumab for Metastatic Colorectal Cancer: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial
- PMID: 32372960
- PMCID: PMC7187887
- DOI: 10.3389/fphar.2020.00478
Traditional Chinese Medicine Combined With Chemotherapy and Cetuximab or Bevacizumab for Metastatic Colorectal Cancer: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial
Abstract
Background: Huangci Granule is a traditional Chinese medicine for treating metastatic colorectal cancer (mCRC).
Objective: To evaluate the efficacy and safety of Huangci Granule combination with chemotherapy and cetuximab (CET) or bevacizumab (BV) for treating mCRC.
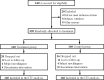
Methods: We performed a randomized, controlled, and double-blind trial and recruited patients with mCRC who were planned to undergo chemotherapy combined with CET or BV. The treatment group was treated with Huangci Granule, while the control group was treated with placebo. Continuous treatment until disease progression, death, intolerable toxicity or up to 6 months. The primary endpoint was progression-free survival (PFS), and the secondary endpoint was quality of life and safety.
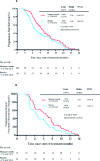
Result: 320 patients were randomly assigned to receive treatment, including 200 first-line patients and 120 second-line patients. In the first-line treatment, the median PFS was 9.59 months (95% CI, 6.94-13.25) vs 6.89 months (95% CI, 4.99-9.52) in treatment group and control group (HR, 0.69; 95% CI, 0.50-0.97; P = 0.027). Chinese medicine was an independent factor affecting the PFS. In the second-line treatment, the median PFS was 6.51 months (95% CI, 4.49-9.44) vs 4.53 months (95% CI, 3.12-6.57) in the treatment group and control group (HR, 0.65; 95% CI, 0.45-0.95; P = 0.020). Compared with the control group, "role function," "social function," "fatigue," and "appetite loss" were significantly improved in the treatment (P < 0.05) and drug related grades 3 to 4 adverse events were less.
Conclusion: Huangci Granule combined with chemotherapy and CET or BV can prolong the PFS of mCRC, improve the quality of life, reduce adverse reactions, and have good safety.
Keywords: cetuximab (CET) or bevacizumab (BV); metastatic colorectal cancer; progression-free survival; quality of life; traditional Chinese medicine.
Copyright © 2020 Liu, Wu, Jia, Cai, Wang, Zhou, Ji, Sui, Zeng, Xiao, Liu, Huo, Feng, Deng and Li.
Figures


References
LinkOut - more resources
Full Text Sources

