Role of Brown Adipose Tissue in Adiposity Associated With Narcolepsy Type 1
- PMID: 32373062
- PMCID: PMC7176868
- DOI: 10.3389/fendo.2020.00145
Role of Brown Adipose Tissue in Adiposity Associated With Narcolepsy Type 1
Abstract
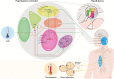
Narcolepsy type 1 is a neurological sleep-wake disorder caused by the destruction of orexin (hypocretin)-producing neurons. These neurons are particularly located in the lateral hypothalamus and have widespread projections throughout the brain, where they are involved, e.g., in the regulation of the sleep-wake cycle and appetite. Interestingly, a higher prevalence of obesity has been reported in patients with narcolepsy type 1 compared to healthy controls, despite a normal to decreased food intake and comparable physical activity. This suggests the involvement of tissues implicated in total energy expenditure, including skeletal muscle, liver, white adipose tissue (WAT), and brown adipose tissue (BAT). Recent evidence from pre-clinical studies with orexin knock-out mice demonstrates a crucial role for the orexin system in the functionality of brown adipose tissue (BAT), probably through multiple pathways. Since BAT is a highly metabolically active organ that combusts fatty acids and glucose toward heat, thereby contributing to energy metabolism, this raises the question of whether BAT plays a role in the development of obesity and related metabolic diseases in narcolepsy type 1. BAT is densely innervated by the sympathetic nervous system that activates BAT, for instance, following cold exposure. The sympathetic outflow toward BAT is mainly mediated by the dorsomedial, ventromedial, arcuate, and paraventricular nuclei in the hypothalamus. This review focuses on the current knowledge on the role of the orexin system in the control of energy balance, with specific focus on BAT metabolism and adiposity in both preclinical and clinical studies.
Keywords: brown adipose tissue; energy metabolism; hypothalamus; narcolepsy type 1; obesity; orexin; sympathetic nervous system.
Copyright © 2020 Straat, Schinkelshoek, Fronczek, Lammers, Rensen and Boon.
Figures
References
-
- Daniels L. Narcolepsy. Medicine. (1934) 13:1–122. 10.1097/00005792-193413010-00001 - DOI
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources

