Mutation of Isocitrate Dehydrogenase 1 in Cholangiocarcinoma Impairs Tumor Progression by Inhibiting Isocitrate Metabolism
- PMID: 32373065
- PMCID: PMC7187788
- DOI: 10.3389/fendo.2020.00189
Mutation of Isocitrate Dehydrogenase 1 in Cholangiocarcinoma Impairs Tumor Progression by Inhibiting Isocitrate Metabolism
Abstract
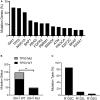
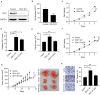
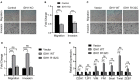
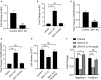
Aim: Isocitrate dehydrogenase 1 (IDH1) is key enzyme involved in cellular metabolism and DNA repair. Mutations in IDH1 occur in up to 25% of cholangiocarcinomas. The present study aimed to explore the features of cellosaurus REB cells with mutant and wide-type IDH1. Methods: To compare the features of IDH1 knockout and mutation in cholangiocarcinoma, we firstly constructed the IDH1 knockout and IDH1 mutation cell lines. We then evaluated the viability of these cell lines using the cell count assay and MTT assay. Next, we determined cell migration and invasion using the Transwell assay. Additionally, to evaluate the effects of IDH1 on cellular metabolism, the levels of α-ketoglutarate (α-KG) and nicotinamide adenine dinucleotide phosphate (NADPH) were determined using enzyme-linked immunosorbent assay. We then applied ChIPbase dataset to explore the genes that were regulated by IDH1. Results: High frequency of mutated IDH1 was observed in the cholangiocarcinoma and IDH1 R132C was presented in more than 80% of mutations. The results showed that IDH1 knockout decreased cell proliferation, migration and invasion, whereas the overexpression of IDH1 in IDH1 knockout cell line recovered its proliferation, migration and invasion capacities. Additionally, IDH1 mutation reduced the levels of NADPH and α-KG. Furthermore, investigation into the underlying mechanisms revealed that IDH1 overexpression induced the expression of aldehyde dehydrogenase 1 thereby promoting cell proliferation, migration and invasion. Conclusion:IDH1 plays an important role in cholangiocarcinoma and its mutation impairs tumor progression in part by inhibition of isocitrate metabolism.
Keywords: NADPH; aldehyde dehydrogenase 1; cholangiocarcinoma; isocitrate dehydrogenase 1; α-ketoglutarate.
Copyright © 2020 Su, Zhang, Zheng, Wang, Zhu and Li.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

