Animal Models of ANCA Associated Vasculitis
- PMID: 32373109
- PMCID: PMC7179669
- DOI: 10.3389/fimmu.2020.00525
Animal Models of ANCA Associated Vasculitis
Abstract
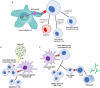
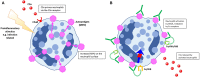
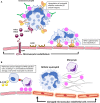
Anti-neutrophil cytoplasmic antibody (ANCA) associated vasculitis (AAV) is a rare and severe autoimmune multisystemic disease. Its pathogenesis involves multiple arms of the immune system, as well as complex interactions between immune cells and target organs. Experimental animal models of disease can provide the crucial link from human disease to translational research into new therapies. This is particularly true in AAV, due to low disease incidence and substantial disease heterogeneity. Animal models allow for controlled environments in which disease mechanisms can be defined, without the clinical confounders of environmental and lifestyle factors. To date, multiple animal models have been developed, each of which shed light on different disease pathways. Results from animal studies of AAV have played a crucial role in enhancing our understanding of disease mechanisms, and have provided direction toward newer targeted therapies. This review will summarize our understanding of AAV pathogenesis as has been gleaned from currently available animal models, as well as address their strengths and limitations. We will also discuss the potential for current and new animal models to further our understanding of this important condition.
Keywords: animal models; antineutrophil cytoplasmic; autoantibodies; autoimmunity; glomerulonephritis; myeloperoxidase; proteinase 3; translational medical research.
Copyright © 2020 Shochet, Holdsworth and Kitching.
Figures
References
-
- Cordova-Sanchez BM, Mejia-Vilet JM, Morales-Buenrostro LE, Loyola-Rodriguez G, Uribe-Uribe NO, Correa-Rotter R. Clinical presentation and outcome prediction of clinical, serological, and histopathological classification schemes in ANCA-associated vasculitis with renal involvement. Clin Rheumatol. (2016) 35:1805–16. 10.1007/s10067-016-3195-z - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

