Bacteria-Induced Acute Inflammation Does Not Reduce the Long-Term Reconstitution Capacity of Bone Marrow Hematopoietic Stem Cells
- PMID: 32373117
- PMCID: PMC7179742
- DOI: 10.3389/fimmu.2020.00626
Bacteria-Induced Acute Inflammation Does Not Reduce the Long-Term Reconstitution Capacity of Bone Marrow Hematopoietic Stem Cells
Abstract
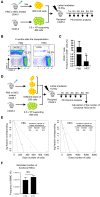
Pathogen-initiated chronic inflammation or autoimmune diseases accelerate proliferation and promote differentiation of hematopoietic stem cells (HSCs) but simultaneously reduce reconstitution capacity. Nevertheless, the effect of acute infection and inflammation on functional HSCs is still largely unknown. Here we found that acute infection elicited by heat-inactivated Escherichia coli (HIEC) expanded bone marrow lineage-negative (Lin)- stem-cell antigen 1 (Sca-1)+cKit+ (LSK) cell population, leading to reduced frequency of functional HSCs in LSK population. However, the total number of BM phenotypic HSCs (Flk2-CD48-CD150+ LSK cells) was not altered in HIEC-challenged mice. Additionally, the reconstitution capacity of the total BM between infected and uninfected mice was similar by both the competitive repopulation assay and measurement of functional HSCs by limiting dilution. Thus, occasionally occurring acute inflammation, which is critical for host defenses, is unlikely to affect HSC self-renewal and maintenance of long-term reconstitution capacity. During acute bacterial infection and inflammation, the hematopoietic system can replenish hematopoietic cells consumed in the innate inflammatory response by accelerating hematopoietic stem and progenitor cell proliferation, but preserving functional HSCs in the BM.
Keywords: acute infection; hematopoietic stem cells; inflammation; long-term reconstitution; self-renewal.
Copyright © 2020 Zhang, Karatepe, Chiewchengchol, Zhu, Guo, Liu, Yu, Ren, Luo, Cheng, Ma, Xu, Han and Luo.
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

