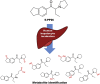
Metabolic profiling of four synthetic stimulants, including the novel indanyl-cathinone 5-PPDi, after human hepatocyte incubation
- PMID: 32373386
- PMCID: PMC7192961
- DOI: 10.1016/j.jpha.2019.12.006
Metabolic profiling of four synthetic stimulants, including the novel indanyl-cathinone 5-PPDi, after human hepatocyte incubation
Abstract
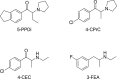
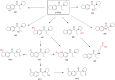
Synthetic cathinones are new psychoactive substances that represent a health risk worldwide. For most of the 130 reported compounds, information about toxicology and/or metabolism is not available, which hampers their detection (and subsequent medical treatment) in intoxication cases. The principles of forensic analytical chemistry and the use of powerful analytical techniques are indispensable for stablishing the most appropriate biomarkers for these substances. Human metabolic fate of synthetic cathinones can be assessed by the analysis of urine and blood obtained from authentic consumers; however, this type of samples is limited and difficult to access. In this work, the metabolic behaviour of three synthetic cathinones (4-CEC, 4-CPrC and 5-PPDi) and one amphetamine (3-FEA) has been evaluated by incubation with pooled human hepatocytes and metabolite identification has been performed by high-resolution mass spectrometry. This in vitro approach has previously shown its feasibility for obtaining excretory human metabolites. 4-CEC and 3-FEA were not metabolised, and for 4-CPrC only two minor metabolites were obtained. On the contrary, for the recently reported 5-PPDi, twelve phase I metabolites were elucidated. Up to our knowledge, this is the first metabolic study of an indanyl-cathinone. Data reported in this paper will allow the detection of these synthetic stimulants in intoxication cases, and will facilitate future research on the metabolic behaviour of other indanyl-based cathinones.
Keywords: 5-PPDi; Hepatocyte incubation; High resolution mass spectrometry; In vitro metabolism; Metabolite identification; Synthetic cathinones.
© 2020 Xi'an Jiaotong University. Production and hosting by Elsevier B.V.
Figures
References
-
- European Monitoring Centre for Drugs and Drug Addiction, European Drug Report 2018. EMCDDA Publ; 2018. - DOI
-
- Liu C., Jia W., Li T. Identification and analytical characterization of nine synthetic cathinone derivatives N -ethylhexedrone, 4-Cl-pentedrone, 4-Cl- α -EAPP, propylone, N -ethylnorpentylone, 6-MeO-bk-MDMA, α -PiHP, 4-Cl- α -PHP, and 4-F- α -PHP. Drug Test Anal. 2017;9:1162–1171. doi: 10.1002/dta.2136. - DOI - PubMed
LinkOut - more resources
Full Text Sources

