Configurational Selection in Azobenzene-Based Supramolecular Systems Through Dual-Stimuli Processes
- PMID: 32373423
- PMCID: PMC7197086
- DOI: 10.1002/open.202000045
Configurational Selection in Azobenzene-Based Supramolecular Systems Through Dual-Stimuli Processes
Abstract
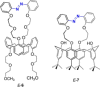
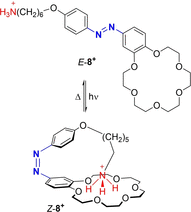
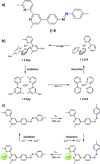
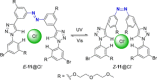
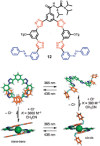
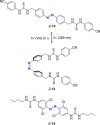
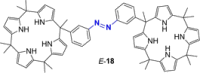
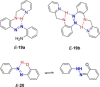
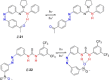
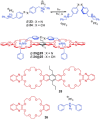
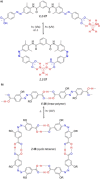
Azobenzene is one of the most studied light-controlled molecular switches and it has been incorporated in a large variety of supramolecular systems to control their structural and functional properties. Given the peculiar isomeric distribution at the photoexcited state (PSS), azobenzene derivatives have been used as photoactive framework to build metastable supramolecular systems that are out of the thermodynamic equilibrium. This could be achieved exploiting the peculiar E/Z photoisomerization process that can lead to isomeric ratios that are unreachable in thermal equilibrium conditions. The challenge in the field is to find molecular architectures that, under given external circumstances, lead to a given isomeric ratio in a reversible and predictable manner, ensuring an ultimate control of the configurational distribution and system composition. By reviewing early and recent works in the field, this review aims at describing photoswitchable systems that, containing an azobenzene dye, display a controlled configurational equilibrium by means of a molecular recognition event. Specifically, examples include programmed photoactive molecular architectures binding cations, anions and H-bonded neutral guests. In these systems the non-covalent molecular recognition adds onto the thermal and light stimuli, equipping the supramolecular architecture with an additional external trigger to select the desired configuration composition.
Keywords: azobenzenes; functional systems; molecular recognition; out-of-the equilibrium; supramolecular chemistry.
© 2020 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



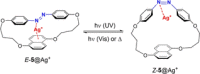






References
-
- Hartley G. S., Nature 1937, 140, 281–282.
-
- Bléger D., Hecht S., Angew. Chem. Int. Ed. 2015, 54, 11338–11349. - PubMed
-
- Baroncini M., Ragazzon G., Silvi S., Venturi M., Credi A., Pure Appl. Chem. 2015, 87, 537–545.
-
- Klajn R., Pure Appl. Chem. 2010, 82, 2247–2279.
Publication types
LinkOut - more resources
Full Text Sources

