Solubilization of Human Interferon β-1b Inclusion Body Proteins by Organic Solvents
- PMID: 32373491
- PMCID: PMC7191233
- DOI: 10.34172/apb.2020.027
Solubilization of Human Interferon β-1b Inclusion Body Proteins by Organic Solvents
Abstract
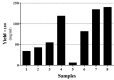
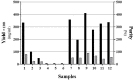
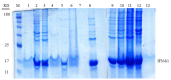
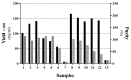
Purposes: Solubilization of inclusion bodies expressed in E. coli is a critical step during manufacturing of recombinant proteins expressed as inclusion bodies. So far, various methods have been used for solubilization and purification of inclusion body proteins to obtain active proteins with high purity and yield. The aim of this study was to examine the benefit of organic solvents such as alcohols in solubilization of recombinant interferon β-1b inclusion bodies. Methods: Effect of important parameters inclusion pH, concentration and type of denaturant and concentration of alcoholic solvents were optimized to formulate a suitable solubilization buffer and investigate their effect on solubilization of interferon β-1b inclusion bodies. Results: Our findings showed the acidic pH in the range of 2-3 is more suitable than alkaline pH >12 for solubilization and achieving higher content of interferon β-1beta and pure recombinant protein. We have also demonstrated that 1% SDS acts better than 2M urea to solubilize Inclusion body proteins of interferon β-1b at pH of 2-3. The interferon concentration was 2.35 mg per 100 mg IB when we used 40% (v/v) 1-propanol and 20% (v/v) 2-butanol into the buffer solution as well. Conclusion: The optimized method provides gentile condition for solubilization of inclusion body at high protein concentration and purity with a degree of retention of native secondary structure which makes this method valuable to be used in production and research area.
Keywords: Alcohol; Inclusion body; Interferon beta-1b; Organic solvent; Solubilization.
© 2020 The Authors.
Figures
Similar articles
-
Recovery of bioactive protein from bacterial inclusion bodies using trifluoroethanol as solubilization agent.Microb Cell Fact. 2016 Jun 8;15:100. doi: 10.1186/s12934-016-0504-9. Microb Cell Fact. 2016. PMID: 27277580 Free PMC article.
-
[Solubilization Specificities Interferon beta-1b from Inclusion Bodies].Bioorg Khim. 2015 Jul-Aug;41(4):403-10. doi: 10.1134/s1068162015040159. Bioorg Khim. 2015. PMID: 26615635 Russian.
-
A single freeze-thawing cycle for highly efficient solubilization of inclusion body proteins and its refolding into bioactive form.Microb Cell Fact. 2015 Feb 22;14:24. doi: 10.1186/s12934-015-0208-6. Microb Cell Fact. 2015. PMID: 25879903 Free PMC article.
-
Protein recovery from inclusion bodies of Escherichia coli using mild solubilization process.Microb Cell Fact. 2015 Mar 25;14:41. doi: 10.1186/s12934-015-0222-8. Microb Cell Fact. 2015. PMID: 25889252 Free PMC article. Review.
-
Isolation and renaturation of bio-active proteins expressed in Escherichia coli as inclusion bodies.Arzneimittelforschung. 1992 Dec;42(12):1512-5. Arzneimittelforschung. 1992. PMID: 1288517 Review.
Cited by
-
Strategic optimization of conditions for the solubilization of GST-tagged amphipathic helix-containing ciliary proteins overexpressed as inclusion bodies in E. coli.Microb Cell Fact. 2022 Dec 12;21(1):258. doi: 10.1186/s12934-022-01979-y. Microb Cell Fact. 2022. PMID: 36510188 Free PMC article.
-
State-of-the-art and novel approaches to mild solubilization of inclusion bodies.Front Bioeng Biotechnol. 2023 Jul 20;11:1249196. doi: 10.3389/fbioe.2023.1249196. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 37545893 Free PMC article. Review.
-
Interferon-Based Biopharmaceuticals: Overview on the Production, Purification, and Formulation.Vaccines (Basel). 2021 Apr 1;9(4):328. doi: 10.3390/vaccines9040328. Vaccines (Basel). 2021. PMID: 33915863 Free PMC article. Review.
References
-
- Isaacs A, Lindenmann J. Pillars Article: Virus Interference I The Interferon Proc R Soc Lond B Biol Sci 1957 147: 258-267. J Immunol. 2015;195(5):1911–20. - PubMed
-
- Faulds D, Benfield P. Interferon beta-1b in multiple sclerosis. Clin Immunother. 1994;1(1):79–87. doi: 10.1007/bf03258493. - DOI
-
- Buraglio M, Trinchard-Lugan I, Munafo A, Macnamee M. Recombinant human interferon-beta-1a (Rebif®) vs recombinant interferon-beta-1b (Betaseron®) in healthy volunteers. Clin Drug Investig. 1999;18(1):27–34. doi: 10.2165/00044011-199918010-00004. - DOI
LinkOut - more resources
Full Text Sources