Fascin Activates β-Catenin Signaling and Promotes Breast Cancer Stem Cell Function Mainly Through Focal Adhesion Kinase (FAK): Relation With Disease Progression
- PMID: 32373510
- PMCID: PMC7186340
- DOI: 10.3389/fonc.2020.00440
Fascin Activates β-Catenin Signaling and Promotes Breast Cancer Stem Cell Function Mainly Through Focal Adhesion Kinase (FAK): Relation With Disease Progression
Abstract
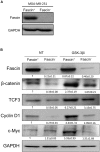
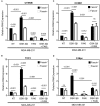
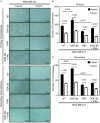
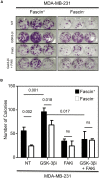
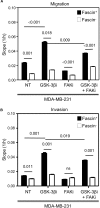
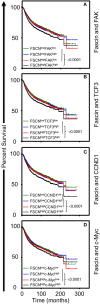
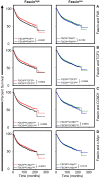
Cancer stem cells (CSCs), a rare population of tumor cells with high self-renewability potential, have gained increasing attention due to their contribution to chemoresistance and metastasis. We have previously demonstrated a critical role for the actin-bundling protein (fascin) in mediating breast cancer chemoresistance through activation of focal adhesion kinase (FAK). The latter is known to trigger the β-catenin signaling pathway. Whether fascin activation of FAK would ultimately trigger β-catenin signaling pathway has not been elucidated. Here, we assessed the effect of fascin manipulation in breast cancer cells on triggering β-catenin downstream targets and its dependence on FAK. Gain and loss of fascin expression showed its direct effect on the constitutive expression of β-catenin downstream targets and enhancement of self-renewability. In addition, fascin was essential for glycogen synthase kinase 3β inhibitor-mediated inducible expression and function of the β-catenin downstream targets. Importantly, fascin-mediated constitutive and inducible expression of β-catenin downstream targets, as well as its subsequent effect on CSC function, was at least partially FAK dependent. To assess the clinical relevance of the in vitro findings, we evaluated the consequence of fascin, FAK, and β-catenin downstream target coexpression on the outcome of breast cancer patient survival. Patients with coexpression of fascinhigh and FAKhigh or high β-catenin downstream targets showed the worst survival outcome, whereas in fascinlow, patient coexpression of FAKhigh or high β-catenin targets had less significant effect on the survival. Altogether, our data demonstrated the critical role of fascin-mediated β-catenin activation and its dependence on intact FAK signaling to promote breast CSC function. These findings suggest that targeting of fascin-FAK-β-catenin axis may provide a novel therapeutic approach for eradication of breast cancer from the root.
Keywords: FAK; breast cancer; cancer stem cell; fascin; β-catenin.
Copyright © 2020 Barnawi, Al-Khaldi, Bakheet, Fallatah, Alaiya, Ghebeh and Al-Alwan.
Figures
References
-
- Yoder BJ, Tso E, Skacel M, Pettay J, Tarr S, Budd T, et al. The expression of fascin, an actin-bundling motility protein, correlates with hormone receptor-negative breast cancer and a more aggressive clinical course. Clin Cancer Res. (2005) 11:186–92. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

