Delivery and Biosafety of Oncolytic Virotherapy
- PMID: 32373515
- PMCID: PMC7176816
- DOI: 10.3389/fonc.2020.00475
Delivery and Biosafety of Oncolytic Virotherapy
Abstract
In recent years, oncolytic virotherapy has emerged as a promising anticancer therapy. Oncolytic viruses destroy cancer cells, without damaging normal tissues, through virus self-replication and antitumor immunity responses, showing great potential for cancer treatment. However, the clinical guidelines for administering oncolytic virotherapy remain unclear. Delivery routes for oncolytic virotherapy to patients vary in existing studies, depending on the tumor sites and the objective of studies. Moreover, the biosafety of oncolytic virotherapy, including mainly uncontrolled adverse events and long-term complications, remains a serious concern that needs to be accurately measured. This review provides a comprehensive and detailed overview of the delivery and biosafety of oncolytic virotherapy.
Keywords: biosafety; delivery route; immunotherapy; oncolytic virotherapy; tumor.
Copyright © 2020 Li, Liu, Han, Tang and Ma.
Figures

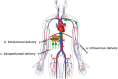
References
-
- Asada T. Treatment of human cancer with mumps virus. Cancer. (1974) 34:1907–28. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

