The Use of a Humanized NSG-β2m-/- Model for Investigation of Immune and Anti-tumor Effects Mediated by the Bifunctional Immunotherapeutic Bintrafusp Alfa
- PMID: 32373533
- PMCID: PMC7186351
- DOI: 10.3389/fonc.2020.00549
The Use of a Humanized NSG-β2m-/- Model for Investigation of Immune and Anti-tumor Effects Mediated by the Bifunctional Immunotherapeutic Bintrafusp Alfa
Erratum in
-
Erratum: The Use of a Humanized NSG-β2m-/- Model for Investigation of Immune and Anti-tumor Effects Mediated by the Bifunctional Immunotherapeutic Bintrafusp Alfa.Front Oncol. 2020 Jul 24;10:1548. doi: 10.3389/fonc.2020.01548. eCollection 2020. Front Oncol. 2020. PMID: 32793505 Free PMC article.
Abstract
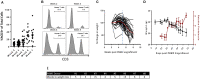
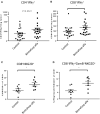
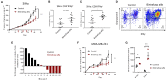
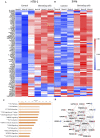
The lack of serial biopsies in patients with a range of carcinomas has been one obstacle in our understanding of the mechanism of action of immuno-oncology agents as well as the elucidation of mechanisms of resistance to these novel therapeutics. While much information can be obtained from studies conducted with syngeneic mouse models, these models have limitations, including that both tumor and immune cells being targeted are murine and that many of the immuno-oncology agents being evaluated are human proteins, and thus multiple administrations are hampered by host xenogeneic responses. Some of these limitations are being overcome by the use of humanized mouse models where human peripheral blood mononuclear cells (PBMC) are engrafted into immunosuppressed mouse strains. Bintrafusp alfa (M7824) is an innovative first-in-class bifunctional fusion protein composed of the extracellular domain of the TGF-βRII to function as a TGF-β "trap" fused to a human IgG1 antibody blocking PD-L1. A phase I clinical trial of bintrafusp alfa showed promising anti-tumor efficacy in heavily pretreated advanced solid tumors, and multiple clinical studies are currently ongoing. There is still much to learn regarding the mechanism of action of bintrafusp alfa, including its effects on both human immune cells in the periphery and in the tumor microenvironment (TME), and any temporal effects upon multiple administrations. By using the NSG-β2m-/- mouse strain humanized with PBMC, we demonstrate here for the first time: (a) the effects of bintrafusp alfa administration on human immune cells in the periphery vs. the TME using three different human xenograft models; (b) temporal effects upon multiple administrations of bintrafusp alfa; (c) phenotypic changes induced in the TME, and (d) variations observed in the use of multiple different PBMC donors. Also discussed are the similarities and differences in the data thus far obtained employing murine syngeneic models, from clinical trials, and in the use of this humanized mouse model. The results described here may guide the future use of this agent or similar immunotherapy agents as monotherapies or in combination therapy studies.
Keywords: NSG-β2m−/−; TGF-β; bintrafusp alfa; humanized mouse; immunotherapy.
Copyright © 2020 Morillon, Smalley Rumfield, Pellom, Sabzevari, Roller, Horn, Jochems, Palena, Greiner and Schlom.
Figures



References
-
- Brehm MA, Kenney LL, Wiles MV, Low BE, Tisch RM, Burzenski L, et al. Lack of acute xenogeneic graft- versus-host disease, but retention of T-cell function following engraftment of human peripheral blood mononuclear cells in NSG mice deficient in MHC class I and II expression. FASEB J. (2019) 33:3137–51. 10.1096/fj.201800636R - DOI - PMC - PubMed
-
- Buchner SM, Sliva K, Bonig H, Volker I, Waibler Z, Kirberg J, et al. Delayed onset of graft-versus-host disease in immunodeficent human leucocyte antigen-DQ8 transgenic, murine major histocompatibility complex class II-deficient mice repopulated by human peripheral blood mononuclear cells. Clin Exp Immunol. (2013) 173:355–64. 10.1111/cei.12121 - DOI - PMC - PubMed
-
- Covassin L, Laning J, Abdi R, Langevin DL, Phillips NE, Shultz LD, et al. Human peripheral blood CD4 T cell-engrafted non-obese diabetic-scid IL2rgamma(null) H2-Ab1 (tm1Gru) Tg (human leucocyte antigen D-related 4) mice: a mouse model of human allogeneic graft-versus-host disease. Clin Exp Immunol. (2011) 166:269–80. 10.1111/j.1365-2249.2011.04462.x - DOI - PMC - PubMed
-
- Pino S, Brehm MA, Covassin-Barberis L, King M, Gott B, Chase TH, et al. Development of novel major histocompatibility complex class I and class II-deficient NOD-SCID IL2R gamma chain knockout mice for modeling human xenogeneic graft-versus-host disease. Methods Mol Biol. (2010) 602:105–17. 10.1007/978-1-60761-058-8_7 - DOI - PMC - PubMed
-
- Yaguchi T, Kobayashi A, Katano I, Ka Y, Ito M, Kawakami Y. MHC class I/II deficient NOG mice are useful for analysis of human T/B cell responses for human tumor immunology research. J ImmunoTher Cancer. (2013) 1(Suppl 1):P39. 10.1186/2051-1426-1-S1-P39 - DOI
LinkOut - more resources
Full Text Sources
Research Materials

