Up-Regulation of Activating Transcription Factor 3 in Human Fibroblasts Inhibits Melanoma Cell Growth and Migration Through a Paracrine Pathway
- PMID: 32373541
- PMCID: PMC7187895
- DOI: 10.3389/fonc.2020.00624
Up-Regulation of Activating Transcription Factor 3 in Human Fibroblasts Inhibits Melanoma Cell Growth and Migration Through a Paracrine Pathway
Abstract
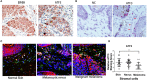
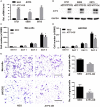
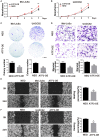
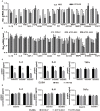
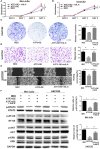
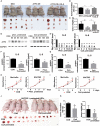
The treatment of melanoma has remained a difficult challenge. Targeting the tumor stroma has recently attracted attention for developing novel strategies for melanoma therapy. Activating transcription factor 3 (ATF3) plays a crucial role in regulating tumorigenesis and development, but whether the expression of ATF3 in human dermal fibroblasts (HDFs) can affect melanoma development hasn't been studied. Our results show that ATF3 expression is downregulated in stromal cells of human melanoma. HDFs expressing high levels of ATF3 suppressed the growth and migration of melanoma cells in association with downregulation of different cytokines including IL-6 in vitro. In vivo, HDFs with high ATF3 expression reduced tumor formation. Adding recombinant IL-6 to melanoma cells reversed those in vitro and in vivo effects, suggesting that ATF3 expression by HDFs regulates melanoma progression through the IL-6/STAT3 pathway. More importantly, HDFs pretreated with cyclosporine A or phenformin to induce ATF3 expression inhibited melanoma cell growth in vitro and in vivo. In summary, our study reveals that ATF3 suppresses human melanoma growth and that inducing the expression of ATF3 in HDFs can inhibit melanoma growth, a new potential melanoma therapeutic approach.
Keywords: activating transcription factor 3; fibroblasts; interleukin 6; melanoma; stromal cells.
Copyright © 2020 Zu, Wen, Xu, Li, Mi, Li, Brakebusch, Fisher and Wu.
Figures
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

