GCN2-Like Kinase Modulates Stress Granule Formation During Nutritional Stress in Trypanosoma cruzi
- PMID: 32373547
- PMCID: PMC7176912
- DOI: 10.3389/fcimb.2020.00149
GCN2-Like Kinase Modulates Stress Granule Formation During Nutritional Stress in Trypanosoma cruzi
Abstract
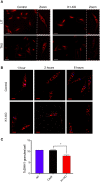
The integrated stress response in eukaryotic cells is an orchestrated pathway that leads to eukaryotic Initiation Factor 2 alpha subunit (eIF2α) phosphorylation at ser51 and ultimately activates pathways to mitigate cellular damages. Three putative kinases (Tck1, Tck2, and Tck3) are found in the Trypanosoma cruzi genome, the flagellated parasite that causes Chagas disease. These kinases present similarities to other eukaryotic eIF2α kinases, exhibiting a typical insertion loop in the kinase domain of the protein. We found that this insertion loop is conserved among kinase 1 of several T. cruzi strains but differs among various Kinetoplastidae species, suggesting unique roles. Kinase 1 is orthologous of GCN2 of several eukaryotes, which have been implicated in the eIF2α ser51 phosphorylation in situations that mainly affects the nutrients levels. Therefore, we further investigated the responses to nutritional stress of T. cruzi devoid of TcK1 generated by CRISPR/Cas9 gene replacement. In nutrient-rich conditions, replicative T. cruzi epimastigotes depleted of TcK1 proliferate as wild type cells but showed increased levels of polysomes relative to monosomes. Upon nutritional deprivation, the polysomes decreased more than in TcK1 depleted line. However, eIF2α is still phosphorylated in TcK1 depleted line, as in wild type parasites. eIF2α phosphorylation increased at longer incubations times, but KO parasites showed less accumulation of ribonucleoprotein granules containing ATP-dependent RNA helicase involved in mRNA turnover (DHH1) and Poly-A binding protein (PABP1). Additionally, the formation of metacyclic-trypomastigotes is increased in the absence of Tck1 compared to controls. These metacyclics, as well as tissue culture trypomastigotes derived from the TcK1 knockout line, were less infective to mammalian host cells, although replicated faster inside mammalian cells. These results indicate that GCN2-like kinase in T. cruzi affects stress granule formation, independently of eIF2α phosphorylation upon nutrient deprivation. It also modulates the fate of the parasites during differentiation, invasion, and intracellular proliferation.
Keywords: CRISPR/Cas9; GCN2; TcK1; Trypanosoma cruzi; eIF2α; phosphorylation; stress-granules.
Copyright © 2020 Malvezzi, Aricó, Souza-Melo, dos Santos, Bittencourt-Cunha, Holetz and Schenkman.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

