Trypanosoma brucei and Trypanosoma cruzi DNA Mismatch Repair Proteins Act Differently in the Response to DNA Damage Caused by Oxidative Stress
- PMID: 32373549
- PMCID: PMC7176904
- DOI: 10.3389/fcimb.2020.00154
Trypanosoma brucei and Trypanosoma cruzi DNA Mismatch Repair Proteins Act Differently in the Response to DNA Damage Caused by Oxidative Stress
Abstract
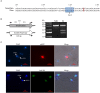
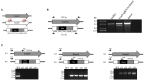
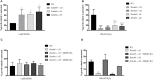
MSH2, associated with MSH3 or MSH6, is a central component of the eukaryotic DNA Mismatch Repair (MMR) pathway responsible for the recognition and correction of base mismatches that occur during DNA replication and recombination. Previous studies have shown that MSH2 plays an additional DNA repair role in response to oxidative damage in Trypanosoma cruzi and Trypanosoma brucei. By performing co-immunoprecipitation followed by mass spectrometry with parasites expressing tagged proteins, we confirmed that the parasites' MSH2 forms complexes with MSH3 and MSH6. To investigate the involvement of these two other MMR components in the oxidative stress response, we generated knockout mutants of MSH6 and MSH3 in T. brucei bloodstream forms and MSH6 mutants in T. cruzi epimastigotes. Differently from the phenotype observed with T. cruzi MSH2 knockout epimastigotes, loss of one or two alleles of T. cruzi msh6 resulted in increased susceptibility to H2O2 exposure, besides impaired MMR. In contrast, T. brucei msh6 or msh3 null mutants displayed increased tolerance to MNNG treatment, indicating that MMR is affected, but no difference in the response to H2O2 treatment when compared to wild type cells. Taken together, our results suggest that, while T. cruzi MSH6 and MSH2 are involved with the oxidative stress response in addition to their role as components of the MMR, the DNA repair pathway that deals with oxidative stress damage operates differently in T. brucei.
Keywords: DNA Mismatch Repair; MSH2; MSH6; Trypanosoma brucei; Trypanosoma cruzi; oxidative stress.
Copyright © 2020 Grazielle-Silva, Zeb, Burchmore, Machado, McCulloch and Teixeira.
Figures


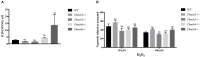
References
Publication types
MeSH terms
Substances
Grants and funding
- BB/M028909/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- G0401553/MRC_/Medical Research Council/United Kingdom
- BB/K006495/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- WT_/Wellcome Trust/United Kingdom
- BB/N016165/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Miscellaneous

