Recent Advances in Ruthenium-Catalyzed Hydrogenation Reactions of Renewable Biomass-Derived Levulinic Acid in Aqueous Media
- PMID: 32373576
- PMCID: PMC7186356
- DOI: 10.3389/fchem.2020.00221
Recent Advances in Ruthenium-Catalyzed Hydrogenation Reactions of Renewable Biomass-Derived Levulinic Acid in Aqueous Media
Abstract
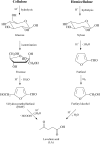
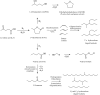
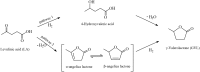
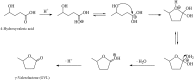
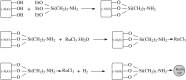
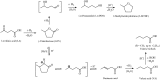
Levulinic acid (LA) is classified as a key platform chemical for the development of future biorefineries, owing to its broad spectrum of potential applications and because it is simply available from lignocellulosic biomass through inexpensive and high-yield production routes. Catalytic hydrogenation reactions of LA into the pivotal intermediate compound γ-valerolactone (GVL), and beyond GVL to yield valeric acid (VA), 1,4-pentanediol (1,4-PDO), and 2-methyltetrahydrofuran (2-MTHF) have gained considerable attention in the last decade. Among the various transition metals used as catalysts in LA hydrogenation reactions, ruthenium-based catalytic systems have been the most extensively applied by far, due to the inherent ability of ruthenium under mild conditions to hydrogenate the keto functionality of LA selectively into an alcohol group to form 4-hydroxyvaleric acid intermediate, which yields GVL spontaneously after dehydration and cyclization. This review focuses on recent advances in the field of aqueous-phase ruthenium-catalyzed hydrogenation reactions of LA toward GVL, VA, 1,4-PDO, 2-MTHF, 2-pentanol, and 2-butanol. It employs heterogeneous catalysts on solid supports, and heterogeneous water-dispersible catalytic nanoparticles or homogeneous water-soluble catalytic complexes with biphasic catalyst separation, for the inter alia production of advanced biofuels such as valeric biofuels and other classes of liquid transportation biofuels, value-added fine chemicals, solvents, additives to gasoline, and to food as well. The significance of the aqueous solvent to carry out catalytic hydrogenations of LA has been highlighted because the presence of water combines several advantages: (i) it is highly polar and thus an ideal medium to convert polar and hydrophilic substrates such as LA; (ii) water is involved as a byproduct; (iii) the presence of the aqueous solvent has a beneficial effect and enormously boosts hydrogenation rates. In sharp contrast, the use of various organic solvents gives rise to a dramatic drop in catalytic activities. The promotional effect of water was proven by numerous experimental investigations and several theoretical studies employing various types of catalytic systems; (iv) the large heat capacity of water renders it an excellent medium to perform large scale exothermic hydrogenations more safely and selectively; and (v) water is a non-toxic, safe, non-inflammable, abundantly available, ubiquitous, inexpensive, and green/sustainable solvent.
Keywords: biofuels; biorefinery; hydrogenation; levulinic acid; platform chemical; renewable; water; γ-valerolactone.
Copyright © 2020 Seretis, Diamantopoulou, Thanou, Tzevelekidis, Fakas, Lilas and Papadogianakis.
Figures
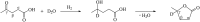
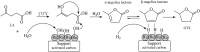


References
-
- Albani D., Li Q., Vilé G., Mitchell S., Almora-Barrios N., Witte P. T., et al. (2017). Interfacial acidity in ligand-modified ruthenium nanoparticles boosts thehydrogenation of levulinic acid to gamma-valerolactone. Green Chem. 19, 2361–2370. 10.1039/C6GC02586B - DOI
-
- Al-Shaal M. G., Wright W. R. H., Palkovits R. (2012). Exploring the ruthenium catalyzedsynthesis of γ-valerolactone in alcohols and utilisation of mild solvent-free reactionconditions. Green Chem. 14, 1260–1263. 10.1039/c2gc16631c - DOI
-
- Aycock D. F. (2007). Solvent applications of 2-methyltetrahydrofuran in organometallic and biphasic reactions. Org. Process Res. Rev. 11, 156–159. 10.1021/op060155c - DOI
-
- Bababrik R. M., Wang B., Resasco D. (2017). Reaction mechanism for the conversion of γ-valerolactone (GVL) over a Ru catalyst: a first-principles study. Ind. Eng. Chem. Res. 56, 3217–3222. 10.1021/acs.iecr.7b00196 - DOI
-
- Badgujar K. C., Badgujar V. C., Bhanage B. M. (2020). A review on catalytic synthesis ofenergy rich fuel additive levulinate compounds from biomass derived levulinic acid. Fuel Process. Technol. 197:106213 10.1016/j.fuproc.2019.106213 - DOI
Publication types
LinkOut - more resources
Full Text Sources

