Quality by design (QbD) approach in processing polymeric nanoparticles loading anticancer drugs by high pressure homogenizer
- PMID: 32373744
- PMCID: PMC7193322
- DOI: 10.1016/j.heliyon.2020.e03846
Quality by design (QbD) approach in processing polymeric nanoparticles loading anticancer drugs by high pressure homogenizer
Abstract
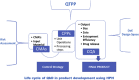
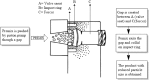
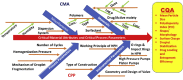
Polymeric nanoparticles prepared using high pressure homogenizer (HPH) present some unique challenges during manufacturing which can be better understood by application of quality by design (QbD) approaches. The present review highlights the ways to identify the critical material attributes which includes the anticancer drugs, polymers, surfactants, solvent system and dispersion system. A comprehensive understanding of the critical processing parameters like pressure and number of cycles during the working of HPH used in putting forward the critical quality attributes such as size, shape, surface charge or droplet stabilization. Such QbD approach will involve development of an effective control strategy for would ensure safe encapsulation of anticancer drugs for successful product development. Proper addressing of the issues related to scaling-up would lead to successful commercialization of the nano-sized formulations loaded with anticancer drugs.
Keywords: Anticancer drugs; Cancer research; High pressure homogenizer; Nanomaterials; Nanotechnology; Oncology; Pharmaceutical science; Polymeric nanoparticles; QbD.
© 2020 Published by Elsevier Ltd.
Figures
References
-
- Doran J., Ryan G. Does nanotechnology research generate an innovation premium over other types of research? Evidence from Ireland. Technol. Soc. 2019;59:101183.
-
- Mukherjee A., Bhattacharyya S. Biotechnology Business-Concept to Delivery. Springer; Cham: 2020. Nanotechnology in medicine; pp. 57–64. 2020.
-
- Soni G., Yadav K.S. Modeling, Methodologies and Tools for Molecular and Nano-Scale Communications. Springer; Cham: 2017. Communication of drug loaded nanogels with cancer cell receptors for targeted delivery; pp. 503–515.
-
- Utreja P., Verma S., Rahman M., Kumar L. Use of nanoparticles in medicine. Curr. Biochem. Eng. 2020;6(1):7–24.
Publication types
LinkOut - more resources
Full Text Sources

