Silencing the cytokine storm: the use of intravenous anakinra in haemophagocytic lymphohistiocytosis or macrophage activation syndrome
- PMID: 32373790
- PMCID: PMC7198216
- DOI: 10.1016/S2665-9913(20)30096-5
Silencing the cytokine storm: the use of intravenous anakinra in haemophagocytic lymphohistiocytosis or macrophage activation syndrome
Abstract
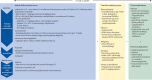
The term cytokine storm syndromes describes conditions characterised by a life-threatening, fulminant hypercytokinaemia with high mortality. Cytokine storm syndromes can be genetic or a secondary complication of autoimmune or autoinflammatory disorders, infections, and haematological malignancies. These syndromes represent a key area of interface between rheumatology and general medicine. Rheumatologists often lead in management, in view of their experience using intensive immunosuppressive regimens and managing cytokine storm syndromes in the context of rheumatic disorders or infection (known as secondary haemophagocytic lymphohistiocytosis or macrophage activation syndrome [sHLH/MAS]). Interleukin (IL)-1 is pivotal in hyperinflammation. Anakinra, a recombinant humanised IL-1 receptor antagonist, is licenced at a dose of 100 mg once daily by subcutaneous injection for rheumatoid arthritis, systemic juvenile idiopathic arthritis, adult-onset Still's disease, and cryopyrin-associated periodic syndromes. In cytokine storm syndromes, the subcutaneous route is often problematic, as absorption can be unreliable in patients with critical illness, and multiple injections are needed to achieve the high doses required. As a result, intravenous anakinra is used in clinical practice for sHLH/MAS, despite this being an off-licence indication and route of administration. Among 46 patients admitted to our three international, tertiary centres for sHLH/MAS and treated with anakinra over 12 months, the intravenous route of delivery was used in 18 (39%) patients. In this Viewpoint, we describe current challenges in the management of cytokine storm syndromes and review the pharmacokinetic and safety profile of intravenous anakinra. There is accumulating evidence to support the rationale for, and safety of, intravenous anakinra as a first-line treatment in patients with sHLH/MAS. Intravenous anakinra has important clinical relevance when high doses of drug are required or if patients have subcutaneous oedema, severe thrombocytopenia, or neurological involvement. Cross-speciality management and collaboration, with the generation of international, multi-centre registries and biobanks, are needed to better understand the aetiopathogenesis and improve the poor prognosis of cytokine storm syndromes.
© 2020 Elsevier Ltd. All rights reserved.
Figures
References
-
- Ramos-Casals M, Brito-Zerón P, López-Guillermo A, Khamashta MA, Bosch X. Adult haemophagocytic syndrome. Lancet. 2014;383:1503–1516. - PubMed
-
- La Rosée P, Horne A, Hines M. Recommendations for the management of hemophagocytic lymphohistiocytosis in adults. Blood. 2019;133:2465–2477. - PubMed
-
- Carter SJ, Tattersall RS, Ramanan AV. Macrophage activation syndrome in adults: recent advances in pathophysiology, diagnosis and treatment. Rheumatology (Oxford) 2019;58:5–17. - PubMed
-
- Kumar B, Aleem S, Saleh H, Petts J, Ballas ZK. A personalized diagnostic and treatment approach for macrophage activation syndrome and secondary hemophagocytic lymphohistiocytosis in adults. J Clin Immunol. 2017;37:638–643. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Medical
Research Materials

