Towards a brain-based predictome of mental illness
- PMID: 32374075
- PMCID: PMC7375108
- DOI: 10.1002/hbm.25013
Towards a brain-based predictome of mental illness
Abstract
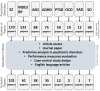
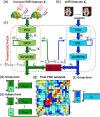
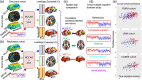
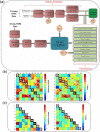
Neuroimaging-based approaches have been extensively applied to study mental illness in recent years and have deepened our understanding of both cognitively healthy and disordered brain structure and function. Recent advancements in machine learning techniques have shown promising outcomes for individualized prediction and characterization of patients with psychiatric disorders. Studies have utilized features from a variety of neuroimaging modalities, including structural, functional, and diffusion magnetic resonance imaging data, as well as jointly estimated features from multiple modalities, to assess patients with heterogeneous mental disorders, such as schizophrenia and autism. We use the term "predictome" to describe the use of multivariate brain network features from one or more neuroimaging modalities to predict mental illness. In the predictome, multiple brain network-based features (either from the same modality or multiple modalities) are incorporated into a predictive model to jointly estimate features that are unique to a disorder and predict subjects accordingly. To date, more than 650 studies have been published on subject-level prediction focusing on psychiatric disorders. We have surveyed about 250 studies including schizophrenia, major depression, bipolar disorder, autism spectrum disorder, attention-deficit hyperactivity disorder, obsessive-compulsive disorder, social anxiety disorder, posttraumatic stress disorder, and substance dependence. In this review, we present a comprehensive review of recent neuroimaging-based predictomic approaches, current trends, and common shortcomings and share our vision for future directions.
Keywords: functional magnetic resonance imaging; machine learning; multimodal studies; neuroimaging; psychiatric disorder.
© 2020 The Authors. Human Brain Mapping published by Wiley Periodicals, Inc.
Conflict of interest statement
The authors declare no conflict of interest related to this work.
Figures












References
-
- Abraham, A. , Milham, M. P. , Di Martino, A. , Craddock, R. C. , Samaras, D. , Thirion, B. , & Varoquaux, G. (2017). Deriving reproducible biomarkers from multi‐site resting‐state data: An autism‐based example. NeuroImage, 147, 736–745. - PubMed
-
- Ad‐Dab'bagh, Y. , Lyttelton, O. , Muehlboeck, J. , Lepage, C. , Einarson, D. , Mok, K. , … Fombonne, E. (2006). The CIVET image‐processing environment: a fully automated comprehensive pipeline for anatomical neuroimaging research. Paper presented at the Proceedings of the 12th annual meeting of the organization for human brain mapping.
-
- Akshoomoff, N. , Lord, C. , Lincoln, A. J. , Courchesne, R. Y. , Carper, R. A. , Townsend, J. , & Courchesne, E. (2004). Outcome classification of preschool children with autism spectrum disorders using MRI brain measures. Journal of the American Academy of Child & Adolescent Psychiatry, 43(3), 349–357. - PubMed
-
- Alain, G. , & Bengio, Y. (2016). Understanding intermediate layers using linear classifier probes. arXiv preprint arXiv:1610.01644.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

