Metabolic changes during pregnancy in glucose-intolerant NZO mice: A polygenic model with prediabetic metabolism
- PMID: 32374082
- PMCID: PMC7201426
- DOI: 10.14814/phy2.14417
Metabolic changes during pregnancy in glucose-intolerant NZO mice: A polygenic model with prediabetic metabolism
Abstract
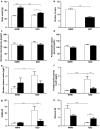
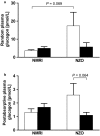
Gestational diabetes mellitus (GDM) is a complex metabolic disease involving genetic and environmental factors. Recent studies have underlined its heterogeneity, so it is reasonable to divide patients into subpopulations depending on whether an insulin secretion or sensitivity defect is predominant. Since testing for GDM is usually performed in the second trimester, misinterpretation of prediabetes as gestational diabetes may occur. As with type 2 diabetes (T2DM), rodent models are needed for both GDM and prediabetes, but few do exist. Here, we compared the metabolic changes in pregnant normal NMRI mice with those in New Zealand obese (NZO) mice. Male animals of this strain are an established model of T2DM, whereas female mice of this strain are protected from hyperglycemia and β-cell death. We demonstrate that female NZO mice exhibited impaired glucose tolerance, preconceptional hyperinsulinemia, and hyperglucagonemia without any signs of manifest diabetes. The NZO model showed, compared with the NMRI control strain, a reduced proliferative response of the Langerhans islets during pregnancy (3.7 ± 0.4 vs. 7.2 ± 0.8% Ki-67-positive nuclei, p = .004). However, oral glucose tolerance tests revealed improved stimulation of insulin secretion in both strains. But this adaption was not sufficient to prevent impaired glucose tolerance in NZO mice compared with the NMRI control (p = .0002). Interestingly, glucose-stimulated insulin secretion was blunted in isolated primary NZO islets in perifusion experiments. In summary, the NZO mouse reflects important characteristics of human GDM and prediabetes in pregnancy and serves as a model for subpopulations with early alterations in glucose metabolism and primary insulin secretion defect.
Keywords: impaired glucose tolerance; polygenic mouse model; prediabetes; pregnancy.
© 2020 The Authors. Physiological Reports published by Wiley Periodicals LLC on behalf of The Physiological Society and the American Physiological Society.
Conflict of interest statement
All authors declare no conflicts of interest relevant to this work.
Figures





References
-
- Ahlqvist, E. , Storm, P. , Käräjämäki, A. , Martinell, M. , Dorkhan, M. , Carlsson, A. , … Groop, L. (2018). Novel subgroups of adult‐onset diabetes and their association with outcomes: A data‐driven cluster analysis of six variables. The Lancet Diabetes and Endocrinology., 6(5), 361–369. 10.1016/S2213-8587(18)30051-2 - DOI - PubMed
-
- American Diabetes Association . (2019). Classification and diagnosis of diabetes: Standards of medical care in diabetes—2019. Diabetes Care, 42(Supplement 1), S13–S28. - PubMed
-
- Bozkurt, L. , Göbl, C. S. , Pfligl, L. , Leitner, K. , Bancher‐Todesca, D. , Luger, A. , … Kautzky‐Willer, A. (2015). Pathophysiological characteristics and effects of obesity in women with early and late manifestation of gestational diabetes diagnosed by the international association of diabetes and pregnancy study groups criteria. The Journal of Clinical Endocrinology and Metabolism, 100(3), 1113–1120. 10.1210/jc.2014-4055 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

