HDAC inhibitor protects chronic cerebral hypoperfusion and oxygen-glucose deprivation injuries via H3K14 and H4K5 acetylation-mediated BDNF expression
- PMID: 32374084
- PMCID: PMC7299713
- DOI: 10.1111/jcmm.15358
HDAC inhibitor protects chronic cerebral hypoperfusion and oxygen-glucose deprivation injuries via H3K14 and H4K5 acetylation-mediated BDNF expression
Abstract
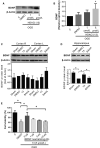
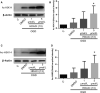
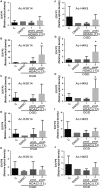
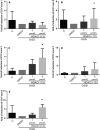
Vascular dementia (VaD) is the second most common cause of dementia, but the treatment is still lacking. Although many studies have reported that histone deacetylase inhibitors (HDACis) confer protective effects against ischemic and hypoxic injuries, their role in VaD is still uncertain. Previous studies shown, one HDACi protected against cognitive decline in animals with chronic cerebral hypoperfusion (CCH). However, the underlying mechanisms remain elusive. In this study, we tested several 10,11-dihydro-5H-dibenzo[b,f]azepine hydroxamates, which act as HDACis in the CCH model (in vivo), and SH-SY5Y (neuroblastoma cells) with oxygen-glucose deprivation (OGD, in vitro). We identified a compound 13, which exhibited the best cell viability under OGD. The compound 13 could increase, in part, the protein levels of brain-derived neurotrophic factor (BDNF). It increased acetylation status on lysine 14 residue of histone 3 (H3K14) and lysine 5 of histone 4 (H4K5). We further clarified which promoters (I, II, III, IV or IX) could be affected by histone acetylation altered by compound 13. The results of chromatin immunoprecipitation and Q-PCR analysis indicate that an increase in H3K14 acetylation leads to an increase in the expression of BDNF promoter II, while an increase in H4K5 acetylation results in an increase in the activity of BDNF promoter II and III. Afterwards, these cause an increase in the expression of BDNF exon II, III and coding exon IX. In summary, the HDACi compound 13 may increase BDNF specific isoforms expression to rescue the ischemic and hypoxic injuries through changes of acetylation on histones.
Keywords: HDAC; OGD; histone acetylation; histone deacetylase inhibitor; vascular dementia.
© 2020 The Authors. Journal of Cellular and Molecular Medicine. published by Foundation for Cellular and Molecular Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Figures


References
-
- Duncombe J, Kitamura A, Hase Y, Ihara M, Kalaria RN, Horsburgh K. Chronic cerebral hypoperfusion: a key mechanism leading to vascular cognitive impairment and dementia. Closing the translational gap between rodent models and human vascular cognitive impairment and dementia. Clin Sci. 2017;131:2451‐2468. - PubMed
-
- Jinglong T, Weijuan G, Jun L, Tao Q, Hongbo Z, Shasha L. The molecular and electrophysiological mechanism of buyanghuanwu decoction in learning and memory ability of vascular dementia rats. Brain Res Bull. 2013;99:13‐18. - PubMed
-
- Ueno M. Elucidation of mechanism of blood‐brain barrier damage for prevention and treatment of vascular dementia. Rinsho Shinkeigaku (Clin Neurol). 2017;57:95‐109. - PubMed
-
- Zhou WB, Lin L, Li ZY, et al. Study on active ingredient and mechanism in preventing vascular dementia of Tianzhusan coming from Tujia medicine. Zhongguo Zhong Yao Za Zhi. 2015;40:2668‐2673. - PubMed
-
- Kaur H, Prakash A, Medhi B. Drug therapy in stroke: from preclinical to clinical studies. Pharmacology. 2013;92:324‐334. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

