Molecular characterization of frog vocal neurons using constellation pharmacology
- PMID: 32374212
- PMCID: PMC7311724
- DOI: 10.1152/jn.00105.2020
Molecular characterization of frog vocal neurons using constellation pharmacology
Abstract
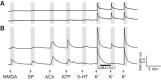
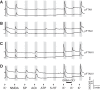
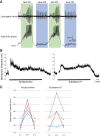
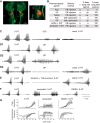
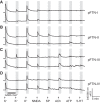
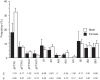
Identification and characterization of neuronal cell classes in motor circuits are essential for understanding the neural basis of behavior. It is a challenging task, especially in a non-genetic-model organism, to identify cell-specific expression of functional macromolecules. Here, we performed constellation pharmacology, calcium imaging of dissociated neurons to pharmacologically identify functional receptors expressed by vocal neurons in adult male and female African clawed frogs, Xenopus laevis. Previously we identified a population of vocal neurons called fast trill neurons (FTNs) in the amphibian parabrachial nucleus (PB) that express N-methyl-d-aspartate (NMDA) receptors and GABA and/or glycine receptors. Using constellation pharmacology, we identified four cell classes of putative fast trill neurons (pFTNs, responsive to both NMDA and GABA/glycine applications). We discovered that some pFTNs responded to the application of substance P (SP), acetylcholine (ACh), or both. Electrophysiological recordings obtained from FTNs using an ex vivo preparation verified that SP and/or ACh depolarize FTNs. Bilateral injection of ACh, SP, or their antagonists into PBs showed that ACh receptors are not sufficient but necessary for vocal production, and SP receptors play a role in shaping the morphology of vocalizations. Additionally, we discovered that the PB of adult female X. laevis also contains all the subclasses of neurons at a similar frequency as in males, despite their sexually distinct vocalizations. These results reveal novel neuromodulators that regulate X. laevis vocal production and demonstrate the power of constellation pharmacology in identifying the neuronal subtypes marked by functional expression of cell-specific receptors in non-genetic-model organisms.NEW & NOTEWORTHY Molecular profiles of neurons are critical for understanding the neuronal functions, but their identification is challenging especially in non-genetic-model organisms. Here, we characterized the functional expression of membrane macromolecules in vocal neurons of African clawed frogs, Xenopus laevis, using a technique called constellation pharmacology. We discovered that receptors for acetylcholine and/or substance P are expressed by some classes of vocal neurons, and their activation plays a role in the production of normal vocalizations.
Keywords: constellation pharmacology; motor programs; parabrachial nucleus; premotor neurons; vocalizations.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures


Similar articles
-
Premotor Neuron Divergence Reflects Vocal Evolution.J Neurosci. 2018 Jun 6;38(23):5325-5337. doi: 10.1523/JNEUROSCI.0089-18.2018. Epub 2018 May 21. J Neurosci. 2018. PMID: 29875228 Free PMC article.
-
Coding rate and duration of vocalizations of the frog, Xenopus laevis.J Neurosci. 2012 Aug 29;32(35):12102-14. doi: 10.1523/JNEUROSCI.2450-12.2012. J Neurosci. 2012. PMID: 22933794 Free PMC article.
-
Rhythm generation, coordination, and initiation in the vocal pathways of male African clawed frogs.J Neurophysiol. 2017 Jan 1;117(1):178-194. doi: 10.1152/jn.00628.2016. Epub 2016 Oct 19. J Neurophysiol. 2017. PMID: 27760822 Free PMC article.
-
Generation, Coordination, and Evolution of Neural Circuits for Vocal Communication.J Neurosci. 2020 Jan 2;40(1):22-36. doi: 10.1523/JNEUROSCI.0736-19.2019. J Neurosci. 2020. PMID: 31896561 Free PMC article. Review.
-
Neuroeffectors for vocalization in Xenopus laevis: hormonal regulation of sexual dimorphism.J Neurobiol. 1986 May;17(3):231-48. doi: 10.1002/neu.480170307. J Neurobiol. 1986. PMID: 3519865 Review.
Cited by
-
Elucidation of Medusozoan (Jellyfish) Venom Constituent Activities Using Constellation Pharmacology.Toxins (Basel). 2024 Oct 17;16(10):447. doi: 10.3390/toxins16100447. Toxins (Basel). 2024. PMID: 39453223 Free PMC article.
-
Emotional vocalizations alter behaviors and neurochemical release into the amygdala.Elife. 2024 Jul 15;12:RP88838. doi: 10.7554/eLife.88838. Elife. 2024. PMID: 39008352 Free PMC article.
References
-
- Bonis JM, Neumueller SE, Krause KL, Kiner T, Smith A, Marshall BD, Qian B, Pan LG, Forster HV. A role for the Kolliker-Fuse nucleus in cholinergic modulation of breathing at night during wakefulness and NREM sleep. J Appl Physiol (1985) 109: 159–170, 2010. doi:10.1152/japplphysiol.00933.2009. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

