Association of Optimal Implementation of Sodium-Glucose Cotransporter 2 Inhibitor Therapy With Outcome for Patients With Heart Failure
- PMID: 32374344
- PMCID: PMC7203666
- DOI: 10.1001/jamacardio.2020.0898
Association of Optimal Implementation of Sodium-Glucose Cotransporter 2 Inhibitor Therapy With Outcome for Patients With Heart Failure
Abstract
Importance: Sodium-glucose cotransporter 2 inhibitor (SGLT2-i) therapy provided incremental survival benefit to patients with heart failure and reduced ejection fraction (HFrEF) who received guideline-directed medical therapy regardless of type 2 diabetes status in a recent clinical trial. To date, estimation of the potential benefits that could be gained from optimal implementation of SGLT2-i therapy at the population level has not been quantified.
Objective: To quantify the projected gains for deaths prevented or postponed with comprehensive implementation of SGLT2-i therapy for patients with HFrEF in the United States.
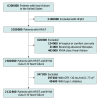
Design, setting, and participants: This decision analytical model, performed from September 25 to October 20, 2019, used published sources to estimate the US population of patients with HFrEF eligible for SGLT2-i therapy and the numbers needed to treat to prevent or postpone overt death. Sensitivity analyses were performed to account for the range of potential benefits.
Main outcomes and measures: All-cause mortality.
Results: Of the 3.1 million patients with HFrEF in the United States, 2 132 800 (69%) were projected to be candidates for SGLT2-i therapy. Optimal implementation of SGLT2-i therapy was empirically estimated to prevent up to 34 125 deaths per year (range 21 840-49 140 deaths per year). A secondary analysis excluding patients on the basis of N-terminal-pro brain natriuretic peptide levels and other trial entry criteria would yield a potential benefit of 25 594 deaths per year prevented (range, 16 380-36 855 deaths per year prevented).
Conclusions and relevance: This study suggests that a substantial number of deaths in the United States could be prevented by optimal implementation of SGLT2-i therapy. These data support implementation of the current evidence into practice in a timely manner to achieve important public health benefits and to reduce the mortality burden of HFrEF.
Conflict of interest statement
Figures