Assessment of Treatment Strategies to Achieve Hepatitis C Elimination in Canada Using a Validated Model
- PMID: 32374397
- PMCID: PMC7203608
- DOI: 10.1001/jamanetworkopen.2020.4192
Assessment of Treatment Strategies to Achieve Hepatitis C Elimination in Canada Using a Validated Model
Abstract
Importance: Achievement of the World Health Organization (WHO) target of eliminating hepatitis C virus (HCV) by 2030 will require an increase in key services, including harm reduction, HCV screening, and HCV treatment initiatives in member countries. These data are not available for Canada but are important for informing a national HCV elimination strategy.
Objective: To use a decision analytical model to explore the association of different treatment strategies with HCV epidemiology and HCV-associated mortality in Canada and to assess the levels of service increase needed to meet the WHO elimination targets by 2030.
Design, setting, and participants: Study participants in this decision analytical model included individuals with hepatitis C virus infection in Canada. Five HCV treatment scenarios (optimistic, very aggressive, aggressive, gradual decrease, and rapid decrease) were applied using a previously validated Markov-type mathematical model. The optimistic and very aggressive treatment scenarios modeled a sustained annual treatment of 10 200 persons and 14 000 persons, respectively, from 2018 to 2030. The aggressive, gradual decrease, and rapid decrease scenarios assessed decreases in treatment uptake from 14 000 persons to 10 000 persons per year, 12 000 persons to 8500 persons per year, and 12 000 persons to 4500 persons per year, respectively, between 2018 and 2030.
Main outcomes and measures: Hepatitis C virus prevalence and HCV-associated health outcomes were assessed for each of the 5 treatment scenarios with the goal of identifying strategies to achieve HCV elimination by 2030.
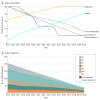
Results: An estimated mean 180 142 persons (95% CI, 122 786-196 862 persons) in Canada had chronic HCV infection at the end of 2017. The optimistic and gradual decrease scenarios estimated a decrease in HCV prevalence from 180 142 persons to 37 246 persons and 37 721 persons, respectively, by 2030. Relative to 2015, this decrease in HCV prevalence was associated with 74%, 69%, and 69% reductions in the prevalence of decompensated cirrhosis, hepatocellular carcinoma, and liver-associated mortality, respectively, leading to HCV elimination by 2030. More aggressive treatment uptake (very aggressive scenario) could result in goal achievement up to 3 years earlier than 2030, although a rapid decrease in the initiation of treatment (rapid decrease scenario) would preclude Canada from reaching the HCV elimination goal by 2030.
Conclusions and relevance: The study findings suggest that Canada could meet the WHO goals for HCV elimination by 2030 by sustaining the current national HCV treatment rate during the next decade. This target will not be achieved if treatment uptake is allowed to decrease rapidly.
Conflict of interest statement
Figures

Comment in
-
Elimination of Hepatitis C Virus Infection in Canada-It Is Achievable, But Is the Rest of the World Ready to Join in the Effort?JAMA Netw Open. 2020 May 1;3(5):e204355. doi: 10.1001/jamanetworkopen.2020.4355. JAMA Netw Open. 2020. PMID: 32374394 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

