Pulmonary pathology of early-phase COVID-19 pneumonia in a patient with a benign lung lesion
- PMID: 32374419
- PMCID: PMC7267508
- DOI: 10.1111/his.14138
Pulmonary pathology of early-phase COVID-19 pneumonia in a patient with a benign lung lesion
Abstract
Aims: An ongoing outbreak of 2019 novel coronavirus (CoV) disease (COVID-19), caused by severe acute respiratory syndrome (SARS) CoV-2, has been spreading in multiple countries. One of the reasons for the rapid spread is that the virus can be transmitted from infected individuals without symptoms. Revealing the pathological features of early-phase COVID-19 pneumonia is important for understanding of its pathogenesis. The aim of this study was to explore the pulmonary pathology of early-phase COVID-19 pneumonia in a patient with a benign lung lesion.
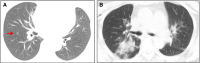
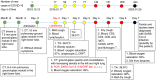
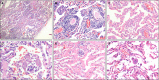
Methods and results: We analysed the pathological changes in lung tissue from a 55-year-old female patient with early-phase SARS-CoV-2 infection. In this case, right lower lobectomy was performed for a benign pulmonary nodule. Detailed clinical, laboratory and radiological data were also examined. This patient was confirmed to have preoperative SARS-CoV-2 infection by the use of real-time reverse transcription polymerase chain reaction and RNA in-situ hybridisation on surgically removed lung tissues. Histologically, COVID-19 pneumonia was characterised by exudative inflammation. The closer to the visceral pleura, the more severe the exudation of monocytes and lymphocytes. Perivascular inflammatory infiltration, intra-alveolar multinucleated giant cells, pneumocyte hyperplasia and intracytoplasmic viral-like inclusion bodies were seen. However, fibrinous exudate and hyaline membrane formation, which were typical pulmonary features of SARS pneumonia, were not evident in this case. Immunohistochemical staining results showed an abnormal accumulation of CD4+ helper T lymphocytes and CD163+ M2 macrophages in the lung tissue.
Conclusion: The results highlighted the pulmonary pathological changes of early-phase SARS-CoV-2 infection, and suggested a role of immune dysfunction in the pathogenesis of COVID-19 pneumonia.
Keywords: 2019 novel coronavirus (SARS-CoV-2); COVID-19 pneumonia; T lymphocyte; macrophage; pathology.
© 2020 John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures




References
-
- Summary of probable SARS cases with onset of illness from 1 November 2002 to 31 July 2003. World Health Organization. Available at: https://www.who.int/csr/sars/country/table2004_04_21/en/ (accessed 8 March 2020).
-
- Middle East respiratory syndrome coronavirus (MERS‐CoV). World Health Organization. Available at: http://www.who.int/emergencies/mers-cov/en/ (accessed 8 March 2020).
-
- Update on the novel coronavirus pneumonia outbreak. China National Health Commission. Available at: http://www.nhc.gov.cn/xcs/yqtb/202004/c5c446c763184509ad8baae9f748ab74.s... (accessed 24 April 2020).
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

