Treatment regimens for administration of anti-vascular endothelial growth factor agents for neovascular age-related macular degeneration
- PMID: 32374423
- PMCID: PMC7202375
- DOI: 10.1002/14651858.CD012208.pub2
Treatment regimens for administration of anti-vascular endothelial growth factor agents for neovascular age-related macular degeneration
Abstract
Background: Age-related macular degeneration (AMD) is one of the leading causes of permanent blindness worldwide. The current mainstay of treatment for neovascular AMD (nAMD) is intravitreal injection of anti-vascular endothelial growth factor (anti-VEGF) agents: aflibercept, ranibizumab, and off-label bevacizumab. Injections can be given monthly, every two or three months ('extended-fixed'), or as needed (pro re nata (PRN)). A variant of PRN is 'treat-and-extend' whereby injections are resumed if recurrence is detected and then delivered with increasing intervals. Currently, injection frequency varies among practitioners, which underscores the need to characterize an optimized approach to nAMD management.
Objectives: To investigate the effects of monthly versus non-monthly intravitreous injection of an anti-VEGF agent in people with newly diagnosed nAMD.
Search methods: We searched CENTRAL, MEDLINE, Embase, LILACS, and three trials registers from 2004 to October 2019; checked references; handsearched conference abstracts; and contacted pharmaceutical companies to identify additional studies.
Selection criteria: We included randomized controlled trials (RCTs) that compared different treatment regimens for anti-VEGF agents in people with newly diagnosed nAMD. We considered standard doses only (ranibizumab 0.5 mg, bevacizumab 1.25 mg, aflibercept 2.0 mg, or a combination of these).
Data collection and analysis: We used standard Cochrane methods for trial selection, data extraction, and analysis.
Main results: We included 15 RCTs. The total number of participants was 7732, ranging from 37 to 2457 in each trial. The trials were conducted worldwide. Of these, six trials exclusively took place in the US, and three included centers from more than one country. Eight trials were at high risk of bias for at least one domain and all trials had at least one domain at unclear risk of bias. Seven trials (3525 participants) compared a PRN regimen with a monthly injection regimen, of which five trials delivered four to eight injections using standard PRN and three delivered nine or 10 injections using a treat-and-extend regimen in the first year. The overall mean change in best-corrected visual acuity (BCVA) at one year was +8.8 letters in the monthly injection group. Compared to the monthly injection, there was moderate-certainty evidence that the mean difference (MD) in BCVA change at one year for the standard PRN subgroup was -1.7 letters (95% confidence interval (CI) -2.8 to -0.6; 4 trials, 2299 participants), favoring monthly injections. There was low-certainty evidence of a similar BCVA change with the treat-and-extend subgroup (0.5 letters, 95% CI -3.1 to 4.2; 3 trials, 1226 participants). Compared to monthly injection, there was low-certainty evidence that fewer participants gained 15 or more lines of vision with standard PRN treatment at one year (risk ratio (RR) 0.87, 95% CI 0.76 to 0.99; 4 trials, 2299 participants) and low-certainty evidence of a similar gain with treat-and-extend versus monthly regimens (RR 1.11, 95% CI 0.91 to 1.36; 3 trials, 1169 participants). The mean change in central retinal thickness was a decrease of -166 μm in the monthly injection group; the MD compared with standard PRN was 21 μm (95% CI 6 to 32; 4 trials, 2215 participants; moderate-certainty evidence) and with treat-and extend was 22 μm (95% CI 37 to -81 μm; 2 trials, 635 participants; low-certainty evidence), in favor of monthly injection. Only one trial (498 participants) measured quality of life and reported no evidence of a difference between regimens, but data could not be extracted (low-certainty evidence). Both PRN regimens (standard and 'treat-and-extend') used fewer injections than monthly regimens (standard PRN: MD -4.6 injections, 95% CI -5.4 to -3.8; 4 trials, 2336 participants; treat-and-extend: -2.4 injections, 95% CI -2.7 to -2.1 injections; moderate-certainty evidence for both comparisons). Two trials provided cost data (1105 participants, trials conducted in the US and the UK). They found that cost differences between regimens were reduced if bevacizumab rather than aflibercept or ranibizumab were used, since bevacizumab was less costly (low-certainty evidence). PRN regimens were associated with a reduced risk of endophthalmitis compared with monthly injections (Peto odds ratio (OR) 0.13, 95% CI 0.04 to 0.46; 6 RCTs, 3175 participants; moderate-certainty evidence). Using data from all trials included in this review, we estimated the risk of endophthalmitis with monthly injections to be 8 in every 1000 people per year. The corresponding risk for people receiving PRN regimens was 1 in every 1000 people per year (95% CI 0 to 4). Three trials (1439 participants) compared an extended-fixed regimen (number of injections reported in only one large trial: 7.5 in one year) with monthly injections. There was moderate-certainty evidence that BCVA at one year was similar for extended-fixed and monthly injections (MD in BCVA change compared to extended-fixed group: -1.3 letters, 95% CI -3.9 to 1.3; RR of gaining 15 letters or more: 0.94, 95% CI 0.80 to 1.10). The change in central retinal thickness was a decrease of 137 μm in the monthly group; the MD with the extended-fixed group was 8 μm (95% CI -11 to 27; low-certainty evidence). The frequency of endophthalmitis was lower in the extended-fixed regimen compared to the monthly group, but this estimate was imprecise (RR 0.19, 95% CI 0.03 to 1.11; low-certainty evidence). If we assumed a risk of 8 cases of endophthalmitis in 1000 people receiving monthly injections over one year, then the corresponding risk with extended-fixed regimen was 2 in 1000 people (95% CI 0 to 9). Other evidence comparing different extended-fixed or PRN regimens yielded inconclusive results.
Authors' conclusions: We found that, at one year, monthly regimens are probably more effective than PRN regimens using seven or eight injections in the first year, but the difference is small and clinically insignificant. Endophthalmitis is probably more common with monthly injections and differences in costs between regimens are higher if aflibercept or ranibizumab are used compared to bevacizumab. This evidence only applies to settings in which regimens are implemented as described in the trials, whereas undertreatment is likely to be common in real-world settings. There are no data from RCTs on long-term effects of different treatment regimens.
Trial registration: ClinicalTrials.gov NCT00593450 NCT00320788 NCT00891735 NCT01306591 NCT01948830 NCT01648292 NCT00509795 NCT02130024.
Copyright © 2020 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
EL: none. SD: none. KL: none. MGK: none. GV: none.
Figures
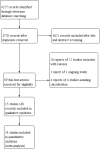





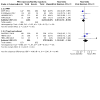


























Update of
References
References to studies included in this review
Barikian 2015a {published data only}
-
- Barikian A, Mahfoud Z, Abdulaal M, Safar A, Bashshur ZF. Induction with intravitreal bevacizumab every two weeks in the management of neovascular age-related macular degeneration. American Journal of Ophthalmology 2015;159(1):121-7. - PubMed
BeMOc 2013 {published data only}
CANTREAT 2019 {published data only (unpublished sought but not used)}
-
- Kertes PJ, Galic IJ, Greve M, Williams RG, Rampakakis E, Scarino A, et al. Canadian treat-and-extend analysis trial with ranibizumab in patients with neovascular age-related macular disease: one-year results of the randomized Canadian treat-and-extend analysis trial with ranibizumab study. Ophthalmology 2019;126(6):841-8. - PubMed
CATT 2011 {published data only}
-
- Chong EW, Al-Qureshi SH. Anti-vascular endothelial growth factor treatment for neovascular age-related macular degeneration: Comparison of Age-related Macular Degeneration Treatments Trials 5 year outcomes and implication for clinical practice. Clinical & Experimental Ophthalmology 2017;45(4):333-5. - PubMed
CLEAR‐IT 2 2011b {published data only}
-
- Brown DM, Heier JS, Ciulla T, Benz M, Abraham P, Yancopoulos G, et al. Primary endpoint results of a phase II study of vascular endothelial growth factor trap-eye in wet age-related macular degeneration. Ophthalmology 2011;118(6):1089-97. - PubMed
-
- Heier JS, Boyer D, Nguyen QD, Marcus D, Roth DB, Yancopoulos G, et al. The 1-year results of CLEAR-IT 2, a phase 2 study of vascular endothelial growth factor trap-eye dosed as-needed after 12-week fixed dosing. Ophthalmology 2011;118(6):1098-106. - PubMed
El‐Mollayess 2012 {published data only}
-
- El-Mollayess GM, Mahfoud Z, Schakal AR, Salti HI, Jaafar D, Bashshur ZF. Fixed-interval versus OCT-guided variable dosing of intravitreal bevacizumab in the management of neovascular age-related macular degeneration: a 12-month randomized prospective study. American Journal of Ophthalmology 2012;153(3):481-9. - PubMed
GMAN 2015 {published data only}
-
- Mahmood S, Roberts SA, Aslam TM, Parkes J, Barugh K, Bishop PN. Routine versus as-needed bevacizumab with 12-weekly assessment intervals for neovascular age-related macular degeneration: 92-week results of the GMAN Trial. Ophthalmology 2015;122(7):1348-55. - PubMed
HARBOR 2013 {published data only}
-
- Busbee BG, Ho AC, Brown DM, Heier JS, Suner IJ, Li Z, et al. Twelve-month efficacy and safety of 0.5 mg or 2.0 mg ranibizumab in patients with subfoveal neovascular age-related macular degeneration. Ophthalmology 2013;120(5):1046-56. - PubMed
-
- Frenkel RE, Fung AE, Shapiro H, Stoilov I. Low luminance vision improves nearly twice as much as standard vision with ranibizumab for neovascular age-related macular degeneration. Investigative Ophthalmology & Visual Science 2015;56(7):5358.
-
- Ho AC, Busbee BG, Regillo CD, Wieland MR, Everen SA, Li Z, et al. Twenty-four-month efficacy and safety of 0.5 mg or 2.0 mg ranibizumab in patients with subfoveal neovascular age-related macular degeneration. Ophthalmology 2014;2014(121):2181-92. - PubMed
-
- Khanani A, Hill L, Tuomi L. Improvement in visual acuity following ranibizumab treatment in neovascular age-related macular degeneration patients with large pigment epithelial detachments: a subgroup analysis of the HARBOR study. Investigative Ophthalmology & Visual Science 2015;56(7):5363.
IVAN 2012b {published data only}ISRCTN92166560
-
- Chakravarthy U, Harding SP, Rogers CA, Downes S, Lotery AJ, Dakin HA, et al. A randomised controlled trial to assess the clinical effectiveness and cost-effectiveness of alternative treatments to Inhibit VEGF in Age-related choroidal Neovascularisation (IVAN). Health Technology Assessment 2015;19:78. - PMC - PubMed
-
- Chakravarthy U, Harding SP, Rogers CA, Downes SM, Lotery AJ, Culliford LA, on behalf of the IVAN study investigators. Alternative treatments to inhibit VEGF in age-related choroidal neovascularisation: 2-year findings of the IVAN randomised controlled trial. Lancet 2013;383(9900):1258-67. - PubMed
-
- Chakravarthy U, Harding SP, Rogers CA, Downes SM, Lotery AJ, Wordsworth S, et al. Ranibizumab versus bevacizumab to treat neovascular age-related macular degeneration: one-year findings from the IVAN randomized trial. Ophthalmology 2012;119(7):1399-411. - PubMed
-
- Lotery A, Scott L, Harding SP, Reeves B, Rogers C, Chakravarthy U. Visual acuity by responder status in the IVAN clinical trial. Investigative Ophthalmology & Visual Science 2014;55(13):867.
Lushchyk 2013 {published data only}
-
- Lushchyk T, Amarakoon S, Martinez-Ciriano JP, Born LI, Baarsma GS, Missotten T. Bevacizumab in age-related macular degeneration: a randomized controlled trial on the effect of injections every 4 weeks, 6 weeks and 8 weeks. Acta Ophthalmologica 2013;91(6):e456-61. - PubMed
NATTB 2012 {published data only}
-
- Li X, Hu Y, Sun X, Zhang J, Zhang M. Neovascular Age-Related Macular Degeneration Treatment Trial Using Bevacizumab (NATTB). Bevacizumab for neovascular age-related macular degeneration in China. Ophthalmology 2012;1119(10):2087-93. - PubMed
Sarraf 2013 {published data only}
-
- Sarraf D, Chan C, Rahimy E, Abraham P. Prospective evaluation of the incidence and risk factors for the development of RPE tears after high- and low-dose ranibizumab therapy. Retina 2013;33(8):1551-7. - PubMed
TREND 2017 {published data only}
-
- Mones J, Silva R, Larsen M, Clemenes A, Muscianisi E, Feller C, et al. Ranibizumab 0.5 mg treat-and-extend versus monthly regimen in neovascular age-related macular degeneration: one-year results from the trend study. Ophthalmic Research 2017;58:3.
-
- Silva R, Berta A, Larsen M, Macfadden W, Feller C, Mones J. Treat-and-extend versus monthly regimen in neovascular age-related macular degeneration: results with ranibizumab from the TREND Study. Ophthalmology 2018;125(1):57-65. - PubMed
TREX‐AMD 2015 {published data only}
-
- Abdelfattah NS, Al-Sheikh M, Pitetta S, Mousa A, Sadda SR, Wykoff CC. Macular atrophy in neovascular age-related macular degeneration with monthly versus treat-and-extend ranibizumab: findings from the TREX-AMD trial. Ophthalmology 2017;124(2):215-23. - PubMed
-
- Wykoff CC, Brown DM, Croft D, Wang R, Clark L, Payne JF. Multicenter, prospective trial comparing treat & extend to monthly dosing in neovascular AMD management: TREXAMD 12-month outcomes. Investigative Ophthalmology & Visual Science 2015;56(7):5371.
-
- Wykoff CC, Croft DE, Brown DM, Wang R, Payne JF, Clark L, et al. Prospective trial of treat-and-extend versus monthly dosing for neovascular age-related macular degeneration: TREX-AMD 1-year results. Ophthalmology 2015;122(12):2514-22. - PubMed
-
- Wykoff CC, Croft DE, Brown DM, Wang R, Payne JF, Clark L, et al. Prospective trial of treat-and-extend versus monthly dosing for neovascular age-related macular degeneration: TREX-AMD 1-year results. Ophthalmology 2015;122(12):2514-22. - PubMed
VIEW 2012 {published data only}
-
- Freund KB, Hoang QV, Saroj N, Thompson D. Intraocular pressure in patients with neovascular age-related macular degeneration receiving intravitreal aflibercept or ranibizumab. Ophthalmology 2015;122(9):1802-10. - PubMed
-
- Heier JS, Brown DM, Chong V, Korobelnik JF, Kaiser PK, Nguyen QD, et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology 2012;119:2537-48. - PubMed
-
- Jaffe GJ, Kaiser PK, Thompson D, Gibson A, Saroj N, Vitti R, et al. Differential response to anti-VEGF regimens in age-related macular degeneration patients with early persistent retinal fluid. Ophthalmology 2016;123(9):1856-64. - PubMed
-
- Korobelnik JF, Morton R, Katz TA, Slakter JS, Sowade O, Wolf S. Baseline characteristics of the fellow eye in patients with wet age-related macular degeneration: post-hoc analysis of the VIEW studies. Investigative Ophthalmology & Visual Science 2014;55(13):3965. - PubMed
-
- Mitchell P, VIEW Study Group. Intravitreal aflibercept versus ranibizumab for neovascular age-related macular degeneration: 52-week subgroup analyses from the combined VIEW studies. Clinical & Experimental Ophthalmology 2012;40(Suppl 1):41-56.
References to studies excluded from this review
Arnold 2015 {published data only}
-
- Arnold J, Markey C, Baird P, Richardson A, Kurstjens N, Guymer R. Baseline characteristics of the fluid study patients: a randomised clinical trial investigating the need for complete resolution of sub-retinal fluid (SRF) in ranibizumab-treated patients with neovascular age-related macular degeneration (NAMD) using a treat and extend (T&E) regimen. Clinical and Experimental Ophthalmology 2015;43:108.
Arnold 2016 {published data only}
Avery 2016 {published data only}
-
- Avery RL. Re: Berg et al.: comparison of ranibizumab and bevacizumab for neovascular age-related macular degeneration according to LUCAS treat-and-extend protocol (Ophthalmology 2015;122:146-52). Ophthalmology 2016;123(2):e14-6. - PubMed
Barikian 2015b {published data only}
-
- Barikian A, Mahfoud Z, Abdulaal M, Safar A, Bashshur ZF. Induction with intravitreal bevacizumab every two weeks in the management of neovascular age-related macular degeneration. American Journal of Ophthalmology 2015;159(1):131-7. - PubMed
Berg 2016 {published data only}
-
- Berg K, Hadzalic E, Gjertsen I, Forsaa V, Berger LH, Kinge B, et al. Ranibizumab or bevacizumab for neovascular age-related macular degeneration according to the Lucentis Compared to Avastin Study Treat-and-Extend Protocol: two-year results. Ophthalmology 2016;123(1):51-9. - PubMed
Bishop 2014 {published data only}
-
- Bishop PN, Roberts S, Aslam T, Charles S, Stanga PE, Parkes J, et al. Randomised controlled trial over 92 weeks using bevacizumab for the treatment of neovascular age-related macular degeneration comparing a treatment regime with 12 weekly regular injections to one with injections on an as-needed basis. Investigative Ophthalmology & Visual Science 2014;55(13):1646.
Eldem 2015 {published data only}
-
- Eldem BM, Muftuoglu G, Topbas S, Cakir M, Kadayifcilar S, Ozmert E, et al. A randomized trial to compare the safety and efficacy of two ranibizumab dosing regimens in a Turkish cohort of patients with choroidal neovascularization secondary to AMD. Acta Ophthalmologica 2015;93(6):e458-64. - PubMed
Enseleit 2017 {published data only}
EXCITE 2011 {published data only}
-
- Golbaz I, Ahlers C, Stock G, Schutze C, Schriefl S, Schlanitz F, et al. Quantification of the therapeutic response of intraretinal, subretinal, and subpigment epithelial compartments in exudative AMD during anti-VEGF therapy. Investigative Ophthalmology & Visual Science 2011;52(3):1599-605. - PubMed
-
- Mayr-Sponer U, Waldstein SM, Kundi M, Ritter M, Golbaz I, Heiling U, et al. Influence of the vitreomacular interface on outcomes of ranibizumab therapy in neovascular age-related macular degeneration. Ophthalmology 2012;120(12):2620-9. - PubMed
-
- Schmidt-Erfurth U, Eldem B, Guymer R, Korobelnik JF, Schlingemann RO, Axer-Siegel R, et al. Efficacy and safety of monthly versus quarterly ranibizumab treatment in neovascular age-related macular degeneration: the EXCITE study. Ophthalmology 2011;118(5):831-9. - PubMed
-
- Simader C, Ritter M, Bolz M, Deak GG, Mayr-Sponer U, Golbaz I, et al. Morphologic parameters relevant for visual outcome during anti-angiogenic therapy of neovascular age-related macular degeneration. Ophthalmology 2014;121(6):1237-45. - PubMed
Feltgen 2014 {published data only}
-
- Feltgen N, Pfeiffer S, Goerlitz A, Hennig H, Bretag M, Neunhoffer H, et al. Efficacy of intravitreal ranibizumab given bimonthly versus PRN in patients with neovascular age-related macular degeneration: 1 year results of a prospective, randomized clinical trial. Investigative Ophthalmology & Visual Science 2014;55(13):3930.
Feltgen 2017 {published data only}
-
- Feltgen N, Bertelmann T, Bretag M, Pfeiffer S, Hilgers R, Callizo J, et al. Efficacy and safety of a fixed bimonthly ranibizumab treatment regimen in eyes with neovascular age-related macular degeneration: results from the RABIMO trial. Albrecht von Graefes Archiv fur Klinische und Experimentelle Ophthalmologie [Graefe's Archive for Clinical and Experimental Ophthalmology] 2017;255(5):923-34. - PubMed
FLUID 2016 {published data only}
-
- Guymer RH, Markey CM, McAllister IL, Gillies MC, Hunyor AP, Arnold JJ, et al. Tolerating subretinal fluid in neovascular age-related macular degeneration treated with ranibizumab using a treat-and-extend regimen: FLUID study 24-month results. Ophthalmology 2019;126(5):723-34. - PubMed
Mahmood 2015 {published data only}
-
- Mahmood S, Roberts SA, Aslam TM, Parkes J, Barugh K, Bishop PN. Routine versus as-needed bevacizumab with 12-weekly assessment intervals for neovascular age-related macular degeneration: 92-week results of the GMAN trial. Ophthalmology 2015;122(7):1348-55. - PubMed
Mori 2017 {published data only}
-
- Mori R, Tanaka K, Haruyama M, Kawamura A, Furuya K, Yuzawa M. Comparison of pro re nata versus bimonthly injection of intravitreal aflibercept for typical neovascular age-related macular degeneration. Ophthalmologica 2017;238(1-2):17-22. - PubMed
RIVAL 2014 {published data only}
-
- Gillies M, Hunyor A, Woodcock C, Walsh M, Kurstjens N. Design and rationale of a clinical trial to measure the development of geographic atrophy in patients undergoing anti-vascular endothelial growth factor treatment for neovascular (WET) age-related macular degeneration: the RIVAL study. 46th Annual Scientific Congress of the Royal Australian and New Zealand College of Ophthalmologists; 2014 Nov 22-26; Brisbane, Australia 2014.
SAVE 2013 {published data only}
-
- Wykoff CC, Brown DM, Chen E, Major JC, Croft DE, Mariani A, et al. SAVE (Super-dose anti-VEGF) trial: 2.0 mg ranibizumab for recalcitrant neovascular age-related macular degeneration: 1-year results. Ophthalmic Surgery, Lasers & Imaging 2013;44(2):121-6. - PubMed
-
- Wykoff CC, Brown DM, Croft DE, Wong TP. Two year SAVE outcomes: 2.0 mg ranibizumab for recalcitrant neovascular AMD. Ophthalmology 2013;120(9):1945-6. - PubMed
SEVEN‐UP 2016 {published data only}
-
- Brijesh T, Shorya A. Macular atrophy progression and 7-year vision outcomes in subjects from the ANCHOR, MARINA, and HORIZON studies: the SEVEN-UP study. American Journal of Ophthalmology 2016;162:200. - PubMed
Takayama 2017 {published data only}
-
- Takayama K, Kaneko H, Sugita T, Maruko R, Hattori K, Ra E, et al. One-year outcomes of 1 + pro re nata versus 3 + pro re nata intravitreal aflibercept injection for neovascular age-related macular degeneration. Ophthalmologica 2017;237(2):105-10. - PubMed
Tempelaar 2015 {published data only}
-
- Tempelaar S, Stephens S, Schouten J. Cost-effectiveness of treatments for wet age-related macular degeneration (WAMD) in the Netherlands, the case of intravitreal aflibercept (IVT-AFL) and ranibizumab. Value in Health 2015;18(7):A420.
Waldstein 2016 {published data only}
-
- Waldstein SM, Wright J, Warburton J, Margaron P, Simader C, Schmidt-Erfurth U. Predictive value of retinal morphology for visual acuity outcomes of different ranibizumab treatment regimens for neovascular AMD. Ophthalmology 2016;123(1):60-9. - PubMed
Wijeyakumar 2015 {published data only}
-
- Wijeyakumar W, Zhu M, Broadhead G, Hong TH, Chang AA. Comparison of aflibercept treatment regimes for treatment resistant neovascular age-related macular degeneration: 8 weekly versus an 'as-needed' regime. Investigative Ophthalmology & Visual Science 2015;56(7):4620.
References to studies awaiting assessment
Kon‐Jara 2007 {published data only}
-
- Kon-Jara V, Torres-Soriano M, Diaz-Rubio J, Hernandez-Rojas M, Guerrero-Naranjo J, Fromow-Guerra J, et al. Efficacy of retreatments with bevacizumab in choroidal neovascularization. Investigative Ophthalmology & Visual Science 2007;48(13):3349.
MATE 2015 {published data only}
-
- Airody A, Dorey T, Mankowska A, Balaskas K, Mukherjee R, Empeslidis T, et al. The MATE study: treating neovascular age related macular degeneration with aflibercept: a pilot, 24 month randomised controlled trial comparing standard care with individualised treat and extend regimen. Investigative Ophthalmology and Visual Science 2018;59(9):ARVO E-abstract 798.
Nunes 2014 {published data only}
-
- Nunes RP, Rodrigues E, Hirai FE, Barroso LF, Novais E, Badaro E, et al. Efficacy and cost-effectiveness of anti-VEGF treatments for age-related macular degeneration (AMD). Investigative Ophthalmology & Visual Science 2014;55(13):4939.
Ohnaka 2017 {published data only}
-
- Ohnaka M, Ohji M, Okada A, Terano Y, Kobayashi M, Takahashi K. Randomised, open-label study to evaluate 2 intravitreal aflibercept treat-and-extend dosing regimens in wet age-related macular degeneration: 52-week outcomes from ALTAIR. 49th Annual Scientific Congress of the Royal Australian and New Zealand College of Ophthalmologists; 2017 Oct 27-Nov 1; Perth, Australia 2017.
References to ongoing studies
Additional references
AAO 2015
-
- American Academy of Ophthalmology. Age-related macular degeneration. eyewiki.aao.org/Age-related_macular_degeneration (accessed 1 September 2015).
Abedi 2014
-
- Abedi F, Wickremasinghe W, Islam AF, Inglis KM, Guymer RH. Anti-VEGF treatment in neovascular age-related macular degeneration. Retina 2014;34(8):1531-8. - PubMed
ANCHOR 2009
-
- Brown DM, Michels M, Kaiser PK, Heier JS, Sy JP, Ianchulev T, et al. Ranibizumab versus verteporfin photodynamic therapy for neovascular age-related macular degeneration: two-year results of the ANCHOR study. Ophthalmology 2009;116(1):57-65. - PubMed
Berg 2017
-
- Berg K, Roald AB, Navaratnam J, Bragadóttir R. An 8-year follow-up of anti-vascular endothelial growth factor treatment with a treat-and-extend modality for neovascular age-related macular degeneration. Acta Ophthalmologica 2017;95:796-802. - PubMed
Bourne 2014
-
- Bourne RR, Jonas JB, Flaxman SR, Keeffe J, Leasher J, Naidoo K, et al. Prevalence and causes of vision loss in high-income countries and in Eastern and Central Europe: 1990-2010. British Journal of Ophthalmology 2014;98(5):629-38. - PubMed
Bressler 2009
-
- Bressler SB. Introduction: understanding the role of angiogenesis and antiangiogenic agents in age-related macular degeneration. Ophthalmology 2009;116(10 Suppl):S1-7. - PubMed
Brown 2005
Brown 2006
-
- Brown MM, Brown GC, Sharma S, Stein JD, Roth Z, Campanella J, et al. The burden of age-related macular degeneration: a value-based analysis. Current Opinion in Ophthalmology 2006;17(3):257-66. - PubMed
Casten 2004
-
- Casten RJ, Rovner BW, Tasman W. Age-related macular degeneration and depression: a review of recent research. Current Opinion in Ophthalmology 2004;15(3):181-3. - PubMed
CATT 2012
-
- Martin DF, Maguire MG, Fine SL, Ying GS, Jaffe GJ, Grunwald JE, et al, Comparison of Age-Related Macular Degeneration Treatments Trial (CATT) Research Group. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmology 2012;119(7):1388-98. - PMC - PubMed
Chin‐Yee 2016
-
- Chin-Yee D, Eck T, Fowler S, Hardi A, Apte RS. A systematic review of as needed versus treat and extend ranibizumab or bevacizumab treatment regimens for neovascular age-related macular degeneration. British Journal of Ophthalmology 2016;100:914-7. - PubMed
Christen 1996
-
- Christen WG, Glynn RJ, Manson JE, Ajani UA, Buring JE. A prospective study of cigarette smoking and risk of age-related macular degeneration in men. JAMA 1996;276(14):1147-51. - PubMed
CLEAR‐IT 2 2011a
-
- Heier JS, Boyer D, Nguyen QD, Marcus D, Roth DB, Yancopoulos G, et al. The 1-year results of CLEAR-IT 2, a phase 2 study of vascular endothelial growth factor trap-eye dosed as-needed after 12-week fixed dosing. Ophthalmology 2011;118(6):1098-106. - PubMed
Covidence [Computer program]
-
- Covidence. Melbourne, Australia: Veritas Health Innovation, accessed 17 January 2019.Available at www.covidence.org.
EuroQol 1990
-
- EuroQol Group. EuroQol – a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199-208. - PubMed
Evans 2005
Ferrara 2004
-
- Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocrine Reviews 2004;25(4):581-611. - PubMed
Fiske 2009
Friberg 2012
-
- Friberg TR, Bilonick RA, Brennen PM. Risk factors for conversion to neovascular age-related macular degeneration based on longitudinal morphologic and visual acuity data. Ophthalmology 2012;119(7):1432-7. - PubMed
Friedman 1999
-
- Friedman DS, Katz J, Bressler NM, Rahmani B, Tielsch JM. Racial differences in the prevalence of age-related macular degeneration: the Baltimore Eye Survey. Ophthalmology 1999;106(6):1049-55. - PubMed
Friedman 2004
-
- Friedman DS, O'Colmain BJ, Munoz B, Tomany SC, McCarty C, Jong PT, et al. Prevalence of age-related macular degeneration in the United States. Archives of Ophthalmology 2004;122(4):564-72. - PubMed
GEFAL 2013
-
- Kodjikian L, Souied EH, Mimoun G, Mauget-Faysse M, Behar-Cohen F, Decullier E, et al. Ranibizumab versus bevacizumab for neovascular age-related macular degeneration: results from the GEFAL non-inferiority randomized trial. Ophthalmology 2013;120(11):2300-9. - PubMed
Glanville 2006
GRADEpro GDT [Computer program]
-
- GRADEpro GDT. Version accessed 5 April 2019. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.Available at gradepro.org.
Gunda 2013
HARBOR 2014
-
- Ho AC, Busbee BG, Regillo CD, Wieland MR, Van Everen SA, Li Z, et al. Twenty-four-month efficacy and safety of 0.5 mg or 2.0 mg ranibizumab in patients with subfoveal neovascular age-related macular degeneration. Ophthalmology 2014;121(11):2181-92. - PubMed
Harper 2010
-
- Harper RA. Chronic visual loss. In: Basic Ophthalmology. 9th edition. San Francisco (CA): American Academy of Ophthalmology, 2010:47-71.
Higgins 2002
-
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Statistics in Medicine 2002;21(11):1539-58. - PubMed
Higgins 2019
-
- Higgins JP, Savović J, Page MJ, Elbers RG, Sterne JA. Chapter 8: Assessing risk of bias in a randomized trial. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
IVAN 2012a
-
- Chakravarthy U, Harding SP, Rogers CA, Downes SM, Lotery AJ, Wordsworth S, et al, Inhibition of VEGF in Age-related choroidal Neovascularization (IVAN) Study Investigators. Ranibizumab versus bevacizumab to treat neovascular age-related macular degeneration: one-year findings from the IVAN randomized trial. Ophthalmology 2012;119(7):1399-411. - PubMed
Kim 2016
-
- Kim LN, Mehta H, Barthelmes D, Nguyen V, Gillies MC. Metaanalysis of real-world outcomes of intravitreal ranibizumab for the treatment of neovascular age-related macular degeneration. Retina 2016;36:1418-31. - PubMed
Klein 1992
-
- Klein R, Klein BE, Linton KL. Prevalence of age-related maculopathy. The Beaver Dam Eye Study. Ophthalmology 1992;99(6):933-43. - PubMed
Leibowitz 1980
-
- Leibowitz HM, Krueger DE, Maunder LR, Milton RC, Kini MM, Kahn HA, et al. The Framingham Eye Study monograph: an ophthalmological and epidemiological study of cataract, glaucoma, diabetic retinopathy, macular degeneration, and visual acuity in a general population of 2631 adults, 1973-1975. Survey of Ophthalmology 1980;24(Suppl):335-610. - PubMed
MANTA 2013
-
- Krebs I, Schmetterer L, Boltz A, Told R, Vecsei-Marlovits V, Egger S, et al. A randomized double-masked trial comparing the visual outcome after treatment with ranibizumab or bevacizumab in patients with neovascular age-related macular degeneration. British Journal of Ophthalmology 2013;97(3):266-71. - PubMed
MARINA 2006
-
- Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, et al. Ranibizumab for neovascular age-related macular degeneration. New England Journal of Medicine 2006;355(14):1419-31. - PubMed
Miller 2013
-
- Miller JW. Age-related macular degeneration revisited – piecing the puzzle: the LXIX Edward Jackson memorial lecture. American Journal of Ophthalmology 2013;155(1):1-35. - PubMed
Moja 2014
NEI VFQ‐25
-
- National Eye Institute. National Eye Institute Visual Functioning Questionnaire-25 (VFQ-25), 2000. nei.nih.gov/sites/default/files/nei-pdfs/vfq_sa.pdf (accessed prior to 28 May 2019).
PIER 2010
-
- Abraham P, Yue H, Wilson L. Randomized, double-masked, sham-controlled trial of ranibizumab for neovascular age-related macular degeneration: PIER study year 2. American Journal of Ophthalmology 2010;150(3):315-24. - PubMed
PrONTO 2009
-
- Lalwani GA, Rosenfeld PJ, Fung AE, Dubovy SR, Michels S, Feuer W, et al. A variable-dosing regimen with intravitreal ranibizumab for neovascular age-related macular degeneration: year 2 of the PrONTO Study. American Journal of Ophthalmology 2009;148(1):43-58. - PubMed
Review Manager 2014 [Computer program]
-
- Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Sarwar 2016
Schmidt‐Erfurth 2011
-
- Schmidt-Erfurth U, Eldem B, Guymer R, Korobelnik JF, Schlingermann RO, Axer-Siegel R, et al. Efficacy and safety of monthly versus quarterly ranibizumab treatment in neovascular age-related macular degeneration: the EXCITE study. Ophthalmology 2011;118(5):831-9. - PubMed
Schmucker 2015
SECURE 2013
-
- Silva R, Axer-Siegel R, Eldem B, Guymer R, Kirchhof B, Papp A, et al. The SECURE Study: long-term safety of ranibizumab 0.5 mg in neovascular age-related macular degeneration. Ophthalmology 2013;120(1):130-9. - PubMed
Seddon 1996
-
- Seddon JM, Willett WC, Speizer FE, Hankinson SE. A prospective study of cigarette smoking and age-related macular degeneration in women. JAMA 1996;276(14):1141-6. - PubMed
Solomon 2014
SUSTAIN 2011
-
- Holz FG, Amoaku W, Donate J, Guymer RH, Kellner U, Schlingermann RO, et al. Safety and efficacy of a flexible dosing regimen of ranibizumab in neovascular age-related macular degeneration: the SUSTAIN study. Ophthalmology 2011;118(4):663-71. - PubMed
Swaroop 2007
-
- Swaroop A, Branham KE, Chen W, Abecasis G. Genetic susceptibility to age-related macular degeneration: a paradigm for dissecting complex disease traits. Human Molecular Genetics 2007;16(R2):R174-82. - PubMed
TAP 2001
-
- Bressler NM, Treatment of Age-Related Macular Degeneration with Photodynamic Therapy (TAP) Study Group. Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin: two-year results of 2 randomized clinical trials-tap report 2. Archives of Ophthalmology 2001;119(2):198-207. - PubMed
VIP 2001
-
- Verteporfin In Photodynamic (VIP) Therapy Study Group. Verteporfin therapy of subfoveal choroidal neovascularization in age-related macular degeneration: two-year results of a randomized clinical trial including lesions with occult with no classic choroidal neovascularization – verteporfin in photodynamic therapy report 2. American Journal of Ophthalmology 2001;131(5):541-60. - PubMed
VISION 2006
-
- Chakravarthy U, Adamis AP, Cunningham ET Jr, Goldbaum M, Guyer DR, Katz B, et al, VEGF Inhibition Study in Ocular Neovascularization (VISION) Clinical Trial Group. Year 2 efficacy results of 2 randomized controlled clinical trials of pegaptanib for neovascular age-related macular degeneration. Ophthalmology 2006;113(9):1508.e1-25. - PubMed
WHO 2016
-
- World Health Organization. Causes of blindness and visual impairment. www.who.int/news-room/fact-sheets/detail/blindness-and-visual-impairment (accessed 12 June 2019).
Wormald 2007
References to other published versions of this review
Li 2016
-
- Li E, Donati S, Virgili G, Lindsley K, Krzystolik MG. Treatment schedules for administration of anti-vascular endothelial growth factor agents for neovascular age-related macular degeneration. Cochrane Database of Systematic Reviews 2016, Issue 5. [DOI: 10.1002/14651858.CD012208] - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

