Seroprevalence of three paramyxoviruses; Hendra virus, Tioman virus, Cedar virus and a rhabdovirus, Australian bat lyssavirus, in a range expanding fruit bat, the Grey-headed flying fox (Pteropus poliocephalus)
- PMID: 32374743
- PMCID: PMC7202650
- DOI: 10.1371/journal.pone.0232339
Seroprevalence of three paramyxoviruses; Hendra virus, Tioman virus, Cedar virus and a rhabdovirus, Australian bat lyssavirus, in a range expanding fruit bat, the Grey-headed flying fox (Pteropus poliocephalus)
Abstract
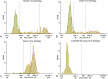
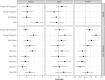
Habitat-mediated global change is driving shifts in species' distributions which can alter the spatial risks associated with emerging zoonotic pathogens. Many emerging infectious pathogens are transmitted by highly mobile species, including bats, which can act as spill-over hosts for pathogenic viruses. Over three years, we investigated the seroepidemiology of paramyxoviruses and Australian bat lyssavirus in a range-expanding fruit bat, the Grey-headed flying fox (Pteropus poliocephalus), in a new camp in Adelaide, South Australia. Over six, biannual, sampling sessions, we quantified median florescent intensity (MFI) antibody levels for four viruses for a total of 297 individual bats using a multiplex Luminex binding assay. Where appropriate, florescence thresholds were determined using finite mixture modelling to classify bats' serological status. Overall, apparent seroprevalence of antibodies directed at Hendra, Cedar and Tioman virus antigens was 43.2%, 26.6% and 95.7%, respectively. We used hurdle models to explore correlates of seropositivity and antibody levels when seropositive. Increased body condition was significantly associated with Hendra seropositivity (Odds ratio = 3.67; p = 0.002) and Hendra virus levels were significantly higher in pregnant females (p = 0.002). While most bats were seropositive for Tioman virus, antibody levels for this virus were significantly higher in adults (p < 0.001). Unexpectedly, all sera were negative for Australian bat lyssavirus. Temporal variation in antibody levels suggests that antibodies to Hendra virus and Tioman virus may wax and wane on a seasonal basis. These findings suggest a common exposure to Hendra virus and other paramyxoviruses in this flying fox camp in South Australia.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
MeSH terms
LinkOut - more resources
Full Text Sources

