Long-term in vivo biocompatibility of single-walled carbon nanotubes
- PMID: 32374764
- PMCID: PMC7202660
- DOI: 10.1371/journal.pone.0226791
Long-term in vivo biocompatibility of single-walled carbon nanotubes
Abstract
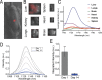
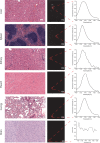
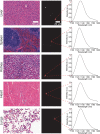
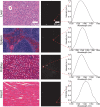
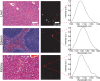
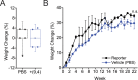
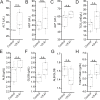
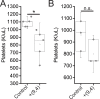
Over the past two decades, measurements of carbon nanotube toxicity and biodistribution have yielded a wide range of results. Properties such as nanotube type (single-walled vs. multi-walled), purity, length, aggregation state, and functionalization, as well as route of administration, greatly affect both the biocompatibility and biodistribution of carbon nanotubes. These differences suggest that generalizable conclusions may be elusive and that studies must be material- and application-specific. Here, we assess the short- and long-term biodistribution and biocompatibility of a single-chirality DNA-encapsulated single-walled carbon nanotube complex upon intravenous administration that was previously shown to function as an in-vivo reporter of endolysosomal lipid accumulation. Regarding biodistribution and fate, we found bulk specificity to the liver and >90% signal attenuation by 14 days in mice. Using near-infrared hyperspectral microscopy to measure single nanotubes, we found low-level, long-term persistence in organs such as the heart, liver, lung, kidney, and spleen. Measurements of histology, animal weight, complete blood count; biomarkers of organ function all suggest short- and long-term biocompatibility. This work suggests that carbon nanotubes can be used as preclinical research tools in-vivo without affecting acute or long-term health.
Conflict of interest statement
D.A.H. is named on a patent filed by MSKCC related to this work (“Composition and method for monitoring lipid”, US patent #10,401,295, issued September 3, 2019). D.A.H. is a cofounder and officer with equity interest of Goldilocks Therapeutics Inc., LipidSense Inc., and Nirova Biosense Inc., as well as a member of the scientific advisory boards of Concarlo Holdings LLC and Nanorobotics Inc. M.A.P. is a cofounder in Repair Ltd. R.E.S. is a member of the scientific advisory board of Miromatrix Inc. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures







References
-
- Lehman JH, Terrones M, Mansfield E, Hurst KE, Meunier V. Evaluating the characteristics of multiwall carbon nanotubes. Carbon. 2011. July 1;49(8):2581–602.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

