Randomized Trial Evaluating Clinical Impact of RAPid IDentification and Susceptibility Testing for Gram-negative Bacteremia: RAPIDS-GN
- PMID: 32374822
- PMCID: PMC8246790
- DOI: 10.1093/cid/ciaa528
Randomized Trial Evaluating Clinical Impact of RAPid IDentification and Susceptibility Testing for Gram-negative Bacteremia: RAPIDS-GN
Abstract
Background: Rapid blood culture diagnostics are of unclear benefit for patients with gram-negative bacilli (GNB) bloodstream infections (BSIs). We conducted a multicenter, randomized, controlled trial comparing outcomes of patients with GNB BSIs who had blood culture testing with standard-of-care (SOC) culture and antimicrobial susceptibility testing (AST) vs rapid organism identification (ID) and phenotypic AST using the Accelerate Pheno System (RAPID).
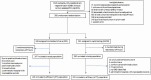
Methods: Patients with positive blood cultures with Gram stains showing GNB were randomized to SOC testing with antimicrobial stewardship (AS) review or RAPID with AS. The primary outcome was time to first antibiotic modification within 72 hours of randomization.
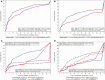
Results: Of 500 randomized patients, 448 were included (226 SOC, 222 RAPID). Mean (standard deviation) time to results was faster for RAPID than SOC for organism ID (2.7 [1.2] vs 11.7 [10.5] hours; P < .001) and AST (13.5 [56] vs 44.9 [12.1] hours; P < .001). Median (interquartile range [IQR]) time to first antibiotic modification was faster in the RAPID arm vs the SOC arm for overall antibiotics (8.6 [2.6-27.6] vs 14.9 [3.3-41.1] hours; P = .02) and gram-negative antibiotics (17.3 [4.9-72] vs 42.1 [10.1-72] hours; P < .001). Median (IQR) time to antibiotic escalation was faster in the RAPID arm vs the SOC arm for antimicrobial-resistant BSIs (18.4 [5.8-72] vs 61.7 [30.4-72] hours; P = .01). There were no differences between the arms in patient outcomes.
Conclusions: Rapid organism ID and phenotypic AST led to faster changes in antibiotic therapy for gram-negative BSIs.
Clinical trials registration: NCT03218397.
Keywords: antibiotic susceptibility testing; blood cultures; bloodstream infection; gram negative; rapid diagnostic.
© The Author(s) 2020. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail: journals.permissions@oup.com.
Figures
References
-
- Albrecht SJ, Fishman NO, Kitchen J, et al. . Reemergence of gram-negative health care-associated bloodstream infections. Arch Intern Med 2006; 166:1289–94. - PubMed
-
- Stryjewski ME, Boucher HW. Gram-negative bloodstream infections. Int J Antimicrob Agents 2009; 34(Suppl 4):S21–5. - PubMed
-
- Vincent JL, Rello J, Marshall J, et al. ; EPIC II Group of Investigators . International study of the prevalence and outcomes of infection in intensive care units. JAMA 2009; 302:2323–9. - PubMed
-
- Ibrahim E, Sherman G, Ward S, Fraser V, Kollef M. The influence of inadequate antimicrobial treatment of bloodstream infections on patient outcomes in the ICU setting. Chest 2000; 118: 46–55. - PubMed
-
- Kang CI, Kim SH, Kim HB, et al. . Pseudomonas aeruginosa bacteremia: risk factors for mortality and influence of delayed receipt of effective antimicrobial therapy on clinical outcome. Clin Infect Dis 2003; 37:745–51. - PubMed

