Low temperature modulates natural peel degreening in lemon fruit independently of endogenous ethylene
- PMID: 32374848
- PMCID: PMC7410192
- DOI: 10.1093/jxb/eraa206
Low temperature modulates natural peel degreening in lemon fruit independently of endogenous ethylene
Abstract
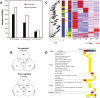
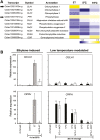
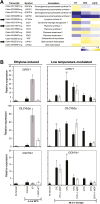
Peel degreening is an important aspect of fruit ripening in many citrus fruit, and previous studies have shown that it can be advanced by ethylene treatment or by low-temperature storage. However, the important regulators and pathways involved in natural peel degreening remain largely unknown. To determine how natural peel degreening is regulated in lemon fruit (Citrus limon), we studied transcriptome and physiochemical changes in the flavedo in response to ethylene treatment and low temperatures. Treatment with ethylene induced rapid peel degreening, which was strongly inhibited by the ethylene antagonist, 1-methylcyclopropene (1-MCP). Compared with 25 ºC, moderately low storage temperatures of 5-20 °C also triggered peel degreening. Surprisingly, repeated 1-MCP treatments failed to inhibit the peel degreening induced by low temperature. Transcriptome analysis revealed that low temperature and ethylene independently regulated genes associated with chlorophyll degradation, carotenoid metabolism, photosystem proteins, phytohormone biosynthesis and signalling, and transcription factors. Peel degreening of fruit on trees occurred in association with drops in ambient temperature, and it coincided with the differential expression of low temperature-regulated genes. In contrast, genes that were uniquely regulated by ethylene showed no significant expression changes during on-tree peel degreening. Based on these findings, we hypothesize that low temperature plays a prominent role in regulating natural peel degreening independently of ethylene in citrus fruit.
Keywords: Citrus limon; 1-methylcyclopropene; carotenoids; chlorophyll; ethylene; low temperature; peel degreening; phytohormones; transcriptome.
© The Author(s) 2020. Published by Oxford University Press on behalf of the Society for Experimental Biology.
Figures







Similar articles
-
Examining the Role of Low Temperature in Satsuma Mandarin Fruit Peel Degreening via Comparative Physiological and Transcriptomic Analysis.Front Plant Sci. 2022 Jul 13;13:918226. doi: 10.3389/fpls.2022.918226. eCollection 2022. Front Plant Sci. 2022. PMID: 35909736 Free PMC article.
-
Altered sensitivity to ethylene in 'Tardivo', a late-ripening mutant of Clementine mandarin.Physiol Plant. 2014 Aug;151(4):507-21. doi: 10.1111/ppl.12133. Epub 2013 Dec 29. Physiol Plant. 2014. PMID: 24372483
-
Involvement of an ethylene response factor in chlorophyll degradation during citrus fruit degreening.Plant J. 2016 Jun;86(5):403-12. doi: 10.1111/tpj.13178. Plant J. 2016. PMID: 27037684
-
Effects of 1-methylcyclopropene (1-MCP) on the expression of genes involved in the chlorophyll degradation pathway of apple fruit during storage.Food Chem. 2020 Mar 5;308:125707. doi: 10.1016/j.foodchem.2019.125707. Epub 2019 Oct 19. Food Chem. 2020. PMID: 31669943
-
Elimination or Removal of Ethylene for Fruit and Vegetable Storage via Low-Temperature Catalytic Oxidation.J Agric Food Chem. 2021 Sep 15;69(36):10419-10439. doi: 10.1021/acs.jafc.1c02868. Epub 2021 Aug 31. J Agric Food Chem. 2021. PMID: 34463513 Review.
Cited by
-
Examining the Role of Low Temperature in Satsuma Mandarin Fruit Peel Degreening via Comparative Physiological and Transcriptomic Analysis.Front Plant Sci. 2022 Jul 13;13:918226. doi: 10.3389/fpls.2022.918226. eCollection 2022. Front Plant Sci. 2022. PMID: 35909736 Free PMC article.
-
Transcriptomic analysis in tomato fruit reveals divergences in genes involved in cold stress response and fruit ripening.Front Plant Sci. 2023 Jul 28;14:1227349. doi: 10.3389/fpls.2023.1227349. eCollection 2023. Front Plant Sci. 2023. PMID: 37575935 Free PMC article.
-
Comparative analysis of the PAL gene family in nine citruses provides new insights into the stress resistance mechanism of Citrus species.BMC Genomics. 2024 Oct 31;25(1):1020. doi: 10.1186/s12864-024-10938-3. BMC Genomics. 2024. PMID: 39482587 Free PMC article.
-
Ethylene Response Factor MaERF012 Modulates Fruit Ripening by Regulating Chlorophyll Degradation and Softening in Banana.Foods. 2022 Dec 1;11(23):3882. doi: 10.3390/foods11233882. Foods. 2022. PMID: 36496689 Free PMC article.
-
The NAC side of the fruit: tuning of fruit development and maturation.BMC Plant Biol. 2021 May 27;21(1):238. doi: 10.1186/s12870-021-03029-y. BMC Plant Biol. 2021. PMID: 34044765 Free PMC article. Review.
References
-
- Agócs A, Nagy V, Szabó Z, Márk L, Ohmacht R, Deli J. 2007. Comparative study on the carotenoid composition of the peel and the pulp of different citrus species. Innovative Food Science and Emerging Technologies 8, 390–394.
-
- Alós E, Cercós M, Rodrigo MJ, Zacarías L, Talón M. 2006. Regulation of color break in citrus fruits. Changes in pigment profiling and gene expression induced by gibberellins and nitrate, two ripening retardants. Journal of Agricultural and Food Chemistry 54, 4888–4895. - PubMed
-
- Asiche WO, Mitalo OW, Kasahara Y, Tosa Y, Mworia EG, Ushijima K, Nakano R, Kubo Y. 2017. Effect of storage temperature on fruit ripening in three kiwifruit cultivars. The Horticulture Journal 86, 403–410.
-
- Barry CS. 2009. The stay-green revolution: recent progress in deciphering the mechanisms of chlorophyll degradation in higher plants. Plant Science 176, 325–333.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

