Respirometric Screening and Characterization of Mitochondrial Toxicants Within the ToxCast Phase I and II Chemical Libraries
- PMID: 32374859
- PMCID: PMC10626520
- DOI: 10.1093/toxsci/kfaa059
Respirometric Screening and Characterization of Mitochondrial Toxicants Within the ToxCast Phase I and II Chemical Libraries
Abstract
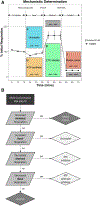
Mitochondrial toxicity drives several adverse health outcomes. Current high-throughput screening assays for chemically induced mitochondrial toxicity typically measure changes to mitochondrial structure and may not detect known mitochondrial toxicants. We adapted a respirometric screening assay (RSA) measuring mitochondrial function to screen ToxCast chemicals in HepG2 cells using a tiered testing strategy. Of 1042 chemicals initially screened at a singlemaximal concentration, 243 actives were identified and rescreened at 7 concentrations. Concentration-response data for 3 respiration phases confirmed activity and indicated a mechanism for 193 mitochondrial toxicants: 149 electron transport chain inhibitors (ETCi), 15 uncouplers and 29 adenosine triphosphate synthase inhibitors. Subsequently, an electron flow assay was used to identify the target complex for 84 of the 149 ETCi. Sixty reference chemicals were used to compare the RSA to existing ToxCast and Tox21 mitochondrial toxicity assays. The RSA was most predictive (accuracy = 90%) of mitochondrial toxicity. The Tox21 mitochondrial membrane potential assay was also highly predictive (accuracy = 87%) of bioactivity but underestimated the potency of well-known ETCi and provided no mechanistic information. The tiered RSA approach accurately identifies and characterizes mitochondrial toxicants acting through diverse mechanisms and at a throughput sufficient to screen large chemical inventories. The electron flow assay provides additional confirmation and detailed mechanistic understanding for ETCi, the most common type of mitochondrial toxicants among ToxCast chemicals. The mitochondrial toxicity screening approach described herein may inform hazard assessment and the in vitro bioactive concentrations used to derive relevant doses for screening level chemical assessment using new approach methodologies.
Keywords: Seahorse; ToxCast; electron transport chain; high-throughput screening; mechanism; mitochondria; new approach methodologies; oxidative phosphorylation; respiration; uncoupling.
Published by Oxford University Press on behalf of the Society of Toxicology 2020. This work is written by US Government employees and is in the public domain in the US.
Conflict of interest statement
DECLARATION OF CONFLICTING INTERESTS
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures





References
-
- Alonso JR, Cardellach F, López S, Casademont J, and Miró O. (2003). Carbon monoxide specifically inhibits cytochrome c oxidase of human mitochondrial respiratory chain. Pharmacol. Toxicol 93, 142–146. - PubMed
-
- Attene-Ramos MS, Huang R, Michael S, Witt KL, Richard A, Tice RR, Simeonov A, Austin CP, and Xia M. (2015). Profiling of the Tox21 chemical collection for mitochondrial function to identify compounds that acutely decrease mitochondrial membrane potential. Environ. Health Perspect 123, 49–56. - PMC - PubMed
-
- Balba H. (2007). Review of strobilurin fungicide chemicals. J. Environ. Sci. Health B 42, 441–451. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

