FVIII half-life extension by coadministration of a D'D3 albumin fusion protein in mice, rabbits, rats, and monkeys
- PMID: 32374879
- PMCID: PMC7218441
- DOI: 10.1182/bloodadvances.2019000999
FVIII half-life extension by coadministration of a D'D3 albumin fusion protein in mice, rabbits, rats, and monkeys
Abstract
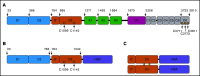
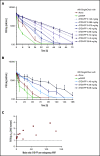
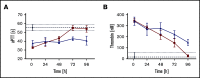
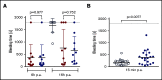
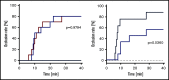
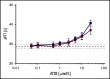
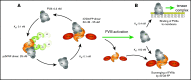
A novel mechanism for extending the circulatory half-life of coagulation factor VIII (FVIII) has been established and evaluated preclinically. The FVIII binding domain of von Willebrand factor (D'D3) fused to human albumin (rD'D3-FP) dose dependently improved pharmacokinetics parameters of coadministered FVIII in all animal species tested, from mouse to cynomolgus monkey, after IV injection. At higher doses, the half-life of recombinant FVIII (rVIII-SingleChain) was calculated to be increased 2.6-fold to fivefold compared with rVIII-SingleChain administered alone in rats, rabbits, and cynomolgus monkeys, and it was increased 3.1-fold to 9.1-fold in mice. Sustained pharmacodynamics effects were observed (ie, activated partial thromboplastin time and thrombin generation measured ex vivo). No increased risk of thrombosis was observed with coadministration of rVIII-SingleChain and rD'D3-FP compared with rVIII-SingleChain alone. At concentrations beyond the anticipated therapeutic range, rD'D3-FP reduced the hemostatic efficacy of coadministered rVIII-SingleChain. This finding might be due to scavenging of activated FVIII by the excessive amount of rD'D3-FP which, in turn, might result in a reduced probability of the formation of the tenase complex. This observation underlines the importance of a fine-tuned balance between FVIII and its binding partner, von Willebrand factor, for hemostasis in general.
© 2020 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: H.L., V.T., J.S., and M.M. are employees of CSL and CSL Behring. S.P., H.-W.B., P.C., E.R., S.K.D., A.A., A.T.-W., S.S., P.M.S., and T.W. are employees and shareholders of CSL and CSL Behring.
Figures

Similar articles
-
Increased potency of recombinant VWF D'D3 albumin fusion proteins engineered for enhanced affinity for coagulation factor VIII.J Thromb Haemost. 2021 Nov;19(11):2710-2725. doi: 10.1111/jth.15480. Epub 2021 Aug 17. J Thromb Haemost. 2021. PMID: 34333849
-
Non-clinical pharmacokinetics and pharmacodynamics of rVIII-SingleChain, a novel recombinant single-chain factor VIII.Thromb Res. 2014 Jul;134(1):125-31. doi: 10.1016/j.thromres.2014.03.028. Epub 2014 Mar 19. Thromb Res. 2014. PMID: 24814969
-
Preclinical efficacy and safety of rVIII-SingleChain (CSL627), a novel recombinant single-chain factor VIII.Thromb Res. 2013 Aug;132(2):280-7. doi: 10.1016/j.thromres.2013.06.017. Epub 2013 Jul 5. Thromb Res. 2013. PMID: 23830969
-
The story of a unique molecule in hemophilia A: recombinant single-chain factor VIII.Thromb Res. 2016 May;141 Suppl 3:S2-4. doi: 10.1016/S0049-3848(16)30414-5. Thromb Res. 2016. PMID: 27288063 Review.
-
Life in the shadow of a dominant partner: the FVIII-VWF association and its clinical implications for hemophilia A.Blood. 2016 Oct 20;128(16):2007-2016. doi: 10.1182/blood-2016-04-713289. Epub 2016 Sep 1. Blood. 2016. PMID: 27587878 Free PMC article. Review.
References
-
- Sanders S, Purcell S, Silva M, Palerme S, James P. Relationship between diagnosis and intervention in women with inherited bleeding disorders and menorrhagia. Haemophilia. 2012;18(3):e273-e276. - PubMed
-
- Astermark J, Petrini P, Tengborn L, Schulman S, Ljung R, Berntorp E. Primary prophylaxis in severe haemophilia should be started at an early age but can be individualized. Br J Haematol. 1999;105(4):1109-1113. - PubMed
-
- Manco-Johnson MJ, Abshire TC, Shapiro AD, et al. . Prophylaxis versus episodic treatment to prevent joint disease in boys with severe hemophilia. N Engl J Med. 2007;357(6):535-544. - PubMed
-
- Tarantino MD, Collins PW, Hay CR, et al. ; RAHF-PFM Clinical Study Group . Clinical evaluation of an advanced category antihaemophilic factor prepared using a plasma/albumin-free method: pharmacokinetics, efficacy, and safety in previously treated patients with haemophilia A. Haemophilia. 2004;10(5):428-437. - PubMed
-
- Mei B, Pan C, Jiang H, et al. . Rational design of a fully active, long-acting PEGylated factor VIII for hemophilia A treatment. Blood. 2010;116(2):270-279. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

