Protective effect of ginsenoside Rk1, a major rare saponin from black ginseng, on cisplatin-induced nephrotoxicity in HEK-293 cells
- PMID: 32374939
- PMCID: PMC11896498
- DOI: 10.1002/kjm2.12220
Protective effect of ginsenoside Rk1, a major rare saponin from black ginseng, on cisplatin-induced nephrotoxicity in HEK-293 cells
Abstract
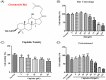
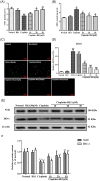
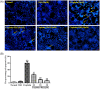
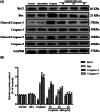
Cisplatin, as one of the most effective chemotherapeutic agents, its clinical use is limited by serious side effect of nephrotoxicity. Cisplatin-induced nephrotoxicity is closely related to apoptosis induction and activation of caspase. The present study aimed to explore the potential protective effect of ginsenoside Rk1 (Rk1), a rare ginsenoside generated during steaming ginseng, on cisplatin-induced nephrotoxicity and the underlying mechanisms in human embryonic kidney 293 (HEK-293) cells. Our results showed that the reduced cell viability induced by cisplatin could significantly recover by Rk1. Furthermore, glutathione (GSH) as an oxidative index, was elevated and the lipid peroxidation product malondialdehyde (MDA) was significantly decreased after Rk1 treatment compared to the cisplatin group. Additionally, Rk1 can also decrease the ROS fluorescence expression and increase the protein levels of nuclear factor erythroid 2-related factor 2 (Nrf2) and heme oxygenase-1 (HO-1) compared to the cisplatin group, which suggested a suppression of oxidative response. More importantly, the cisplatin-induced elevated protein levels of Bax, cleaved caspase-3, cleaved caspase-9, and decreased protein level of Bcl-2 were reversed after treatment with Rk1. Our results elucidated the possible protective mechanism of Rk1 for the first time, which may involve in its anti-oxidation and anti-apoptosis effects.
Keywords: HEK-293; anti-apoptosis; cisplatin; ginsenoside Rk1; nephrotoxicity.
© 2020 The Authors. The Kaohsiung Journal of Medical Sciences published by John Wiley & Sons Australia on behalf of Kaohsiung Medical University.
Conflict of interest statement
The authors declare no conflict of interests.
Figures

Similar articles
-
Ginsenoside Rk1 protects human melanocytes from H2O2‑induced oxidative injury via regulation of the PI3K/AKT/Nrf2/HO‑1 pathway.Mol Med Rep. 2021 Nov;24(5):821. doi: 10.3892/mmr.2021.12462. Epub 2021 Sep 24. Mol Med Rep. 2021. PMID: 34558653 Free PMC article.
-
Protective Effects of Processed Ginseng and Its Active Ginsenosides on Cisplatin-Induced Nephrotoxicity: In Vitro and in Vivo Studies.J Agric Food Chem. 2015 Jul 1;63(25):5964-9. doi: 10.1021/acs.jafc.5b00782. Epub 2015 Jun 19. J Agric Food Chem. 2015. PMID: 26050847
-
Protective effects of two novel nitronyl nitroxide radicals on heart failure induced by hypobaric hypoxia.Life Sci. 2020 May 1;248:116481. doi: 10.1016/j.lfs.2019.05.037. Epub 2019 May 15. Life Sci. 2020. PMID: 31102744
-
Ginsenoside Rg5 Ameliorates Cisplatin-Induced Nephrotoxicity in Mice through Inhibition of Inflammation, Oxidative Stress, and Apoptosis.Nutrients. 2016 Sep 13;8(9):566. doi: 10.3390/nu8090566. Nutrients. 2016. PMID: 27649238 Free PMC article.
-
Protective effect of reduced glutathione C60 derivative against hydrogen peroxide-induced apoptosis in HEK 293T cells.J Huazhong Univ Sci Technolog Med Sci. 2016 Jun;36(3):356-363. doi: 10.1007/s11596-016-1591-x. Epub 2016 Jul 5. J Huazhong Univ Sci Technolog Med Sci. 2016. PMID: 27376803
Cited by
-
Panax ginseng and its ginsenosides: potential candidates for the prevention and treatment of chemotherapy-induced side effects.J Ginseng Res. 2021 Nov;45(6):617-630. doi: 10.1016/j.jgr.2021.03.001. Epub 2021 Mar 5. J Ginseng Res. 2021. PMID: 34764717 Free PMC article. Review.
-
Therapeutic Applications of Ginseng Natural Compounds for Health Management.Int J Mol Sci. 2023 Dec 9;24(24):17290. doi: 10.3390/ijms242417290. Int J Mol Sci. 2023. PMID: 38139116 Free PMC article. Review.
-
Potential therapeutic effects of Chinese meteria medica in mitigating drug-induced acute kidney injury.Front Pharmacol. 2023 Apr 3;14:1153297. doi: 10.3389/fphar.2023.1153297. eCollection 2023. Front Pharmacol. 2023. PMID: 37077810 Free PMC article. Review.
-
Lobetyolin, a Q-marker isolated from Radix Platycodi, exerts protective effects on cisplatin-induced cytotoxicity in HEK293 cells.J Nat Med. 2023 Sep;77(4):721-734. doi: 10.1007/s11418-023-01714-w. Epub 2023 Jun 23. J Nat Med. 2023. PMID: 37353674
-
MicroRNAs Involved in Intrinsic Apoptotic Pathway during Cisplatin-Induced Nephrotoxicity: Potential Use of Natural Products against DDP-Induced Apoptosis.Biomolecules. 2022 Aug 31;12(9):1206. doi: 10.3390/biom12091206. Biomolecules. 2022. PMID: 36139046 Free PMC article. Review.
References
-
- Glassock RJ, Warnock DG, Delanaye P. The global burden of chronic kidney disease: Estimates, variability and pitfalls. Nat Rev Nephrol. 2017;13(2):104–114. - PubMed
-
- Webster AC, Nagler EV, Morton RL, Masson P. Chronic kidney disease. Lancet. 2017;389(10075):1238–1252. - PubMed
-
- Mundhe N, Kumar P, Arora I, Ahmed S, Lahkar M. Differential effect of NDGA on cisplatin‐induced nephrotoxicity in Spargue‐Dawley rats. Immunopharmacol Immunotoxicol. 2019;41(1):68–75. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

