A roadmap for development of neuro-oscillations as translational biomarkers for treatment development in neuropsychopharmacology
- PMID: 32375159
- PMCID: PMC7360555
- DOI: 10.1038/s41386-020-0697-9
A roadmap for development of neuro-oscillations as translational biomarkers for treatment development in neuropsychopharmacology
Abstract
New treatment development for psychiatric disorders depends critically upon the development of physiological measures that can accurately translate between preclinical animal models and clinical human studies. Such measures can be used both as stratification biomarkers to define pathophysiologically homogeneous patient populations and as target engagement biomarkers to verify similarity of effects across preclinical and clinical intervention. Traditional "time-domain" event-related potentials (ERP) have been used translationally to date but are limited by the significant differences in timing and distribution across rodent, monkey and human studies. By contrast, neuro-oscillatory responses, analyzed within the "time-frequency" domain, are relatively preserved across species permitting more precise translational comparisons. Moreover, neuro-oscillatory responses are increasingly being mapped to local circuit mechanisms and may be useful for investigating effects of both pharmacological and neuromodulatory interventions on excitatory/inhibitory balance. The present paper provides a roadmap for development of neuro-oscillatory responses as translational biomarkers in neuropsychiatric treatment development.
Figures
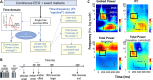
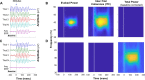
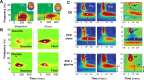
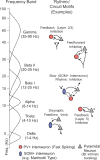
References
-
- Cohen MX. Analyzing neural time series data: theory and practice. Cambridge: MIT press; 2014 .
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

