'All In': a pragmatic framework for COVID-19 testing and action on a global scale
- PMID: 32375201
- PMCID: PMC7267598
- DOI: 10.15252/emmm.202012634
'All In': a pragmatic framework for COVID-19 testing and action on a global scale
Abstract
Current demand for SARS-CoV-2 testing is straining material resource and labor capacity around the globe. As a result, the public health and clinical community are hindered in their ability to monitor and contain the spread of COVID-19. Despite broad consensus that more testing is needed, pragmatic guidance toward realizing this objective has been limited. This paper addresses this limitation by proposing a novel and geographically agnostic framework (the 4Ps framework) to guide multidisciplinary, scalable, resource-efficient, and achievable efforts toward enhanced testing capacity. The 4Ps (Prioritize, Propagate, Partition, and Provide) are described in terms of specific opportunities to enhance the volume, diversity, characterization, and implementation of SARS-CoV-2 testing to benefit public health. Coordinated deployment of the strategic and tactical recommendations described in this framework has the potential to rapidly expand available testing capacity, improve public health decision-making in response to the COVID-19 pandemic, and/or to be applied in future emergent disease outbreaks.
© 2020 The Authors. Published under the terms of the CC BY 4.0 license.
Conflict of interest statement
The views expressed in this paper are those of the authors and do not necessarily reflect the views or policies of the U.S. Environmental Protection Agency, Japan National Institute of Health Sciences, the Pharmaceuticals and Medical Devices Agency, or that of any of the authors’ employers. The authors have no conflicts to disclose.
Figures


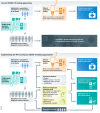
References
-
- Beltran‐Pavez C, Marquez CL, Munoz G, Valiente‐Echeverria F, Gaggero A, Soto‐Rifo R, Barriga GP (2020) SARS‐CoV‐2 detection from nasopharyngeal swab samples without RNA extraction. bioRxiv 10.1101/2020.03.28.013508 [PREPRINT] - DOI
-
- Collinge M, Schneider P, Li D, Parish S, Dumont C, Freebern W, Ghanime J, Guimont‐Derochers F, Langenkamp A, Lebron J et al (2020) Cross‐company evaluation of the human lymphocyte activation assay. J Immunotoxicol, 17: 51–58. - PubMed
-
- Kaplow L (2020) Opinion | Until We Have More Coronavirus Testing Capacity, We Should Test Randomly. New York Times https://www.nytimes.com/2020/04/24/opinion/coronavirus-testing.html?smid...
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

