Glycosylation Tunes Neuroserpin Physiological and Pathological Properties
- PMID: 32375228
- PMCID: PMC7247563
- DOI: 10.3390/ijms21093235
Glycosylation Tunes Neuroserpin Physiological and Pathological Properties
Abstract
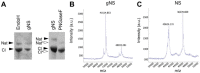
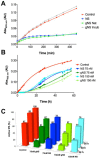
Neuroserpin (NS) is a member of the serine protease inhibitors superfamily. Specific point mutations are responsible for its accumulation in the endoplasmic reticulum of neurons that leads to a pathological condition named familial encephalopathy with neuroserpin inclusion bodies (FENIB). Wild-type NS presents two N-glycosylation chains and does not form polymers in vivo, while non-glycosylated NS causes aberrant polymer accumulation in cell models. To date, all in vitro studies have been conducted on bacterially expressed NS, de facto neglecting the role of glycosylation in the biochemical properties of NS. Here, we report the expression and purification of human glycosylated NS (gNS) using a novel eukaryotic expression system, LEXSY. Our results confirm the correct N-glycosylation of wild-type gNS. The fold and stability of gNS are not altered compared to bacterially expressed NS, as demonstrated by the circular dichroism and intrinsic tryptophan fluorescence assays. Intriguingly, gNS displays a remarkably reduced polymerisation propensity compared to non-glycosylated NS, in keeping with what was previously observed for wild-type NS in vivo and in cell models. Thus, our results support the relevance of gNS as a new in vitro tool to study the molecular bases of FENIB.
Keywords: glycosylation; neuroserpin; protein polymerisation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Hastings G.A., Coleman T.A., Haudenschild C.C., Stefansson S., Smith E.P., Barthlow R., Cherry S., Sandkvist M., Lawrence D.A. Neuroserpin, a brain-associated inhibitor of tissue plasminogen activator is localized primarily in neurons. Implications for the regulation of motor learning and neuronal survival. J. Biol. Chem. 1997;272:33062–33067. doi: 10.1074/jbc.272.52.33062. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

