The Metformin Mechanism on Gluconeogenesis and AMPK Activation: The Metabolite Perspective
- PMID: 32375255
- PMCID: PMC7247334
- DOI: 10.3390/ijms21093240
The Metformin Mechanism on Gluconeogenesis and AMPK Activation: The Metabolite Perspective
Abstract
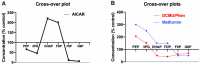
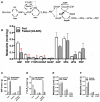
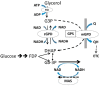
Metformin therapy lowers blood glucose in type 2 diabetes by targeting various pathways including hepatic gluconeogenesis. Despite widespread clinical use of metformin the molecular mechanisms by which it inhibits gluconeogenesis either acutely through allosteric and covalent mechanisms or chronically through changes in gene expression remain debated. Proposed mechanisms include: inhibition of Complex 1; activation of AMPK; and mechanisms independent of both Complex 1 inhibition and AMPK. The activation of AMPK by metformin could be consequent to Complex 1 inhibition and raised AMP through the canonical adenine nucleotide pathway or alternatively by activation of the lysosomal AMPK pool by other mechanisms involving the aldolase substrate fructose 1,6-bisphosphate or perturbations in the lysosomal membrane. Here we review current interpretations of the effects of metformin on hepatic intermediates of the gluconeogenic and glycolytic pathway and the candidate mechanistic links to regulation of gluconeogenesis. In conditions of either glucose excess or gluconeogenic substrate excess, metformin lowers hexose monophosphates by mechanisms that are independent of AMPK-activation and most likely mediated by allosteric activation of phosphofructokinase-1 and/or inhibition of fructose bisphosphatase-1. The metabolite changes caused by metformin may also have a prominent role in counteracting G6pc gene regulation in conditions of compromised intracellular homeostasis.
Keywords: AMPK; Liver metabolism; gluconeogenesis; metformin; phosphofructokinase-1.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

